Now with your cell phone connection,cardiac resynchronization therapy-defibrillator (CRT-D) or implantable cardioverter-defibrillator (ICD) are monitored through the CareLink Network. The cell phone connection is taking over with implants reporting data. I have done several posts over the last couple of years on this subjec t and I still feel it is one of the neglected areas of “meaningful use”. For sure, it’s a difficult area to assess too as it is evolving almost daily. Check out the link below for a summary of some of what is out there.
t and I still feel it is one of the neglected areas of “meaningful use”. For sure, it’s a difficult area to assess too as it is evolving almost daily. Check out the link below for a summary of some of what is out there.
Wearable Sensors and Other Healthcare Innovations Set to Flourish
If you are not implanted, you will be carrying one, wearing one, or sticking one on your body somewhere it seems. Next with aggregation we will see multi purpose devices too, one that may serve 2-3 purposes, and then of course there’s the question of the software being correct with no bugs before we track off into our brave  new world here. 2000 certainly appears to be shaping up as the “decade of the sensors”. Medtronic is ready to get connected with EHRs and EMRs to send the data with their Paceart System to integrate.
new world here. 2000 certainly appears to be shaping up as the “decade of the sensors”. Medtronic is ready to get connected with EHRs and EMRs to send the data with their Paceart System to integrate.
Medtronic has shown the ability to connect with EHR vendors including Epic, General Electric, NextGen, and Medical Micrographics and I’m sure there’s more in the works. You can view a flash presentation here.
After viewing the presentation myself we need some serious software training here in order for this to work for both doctors and patients. What happens when you have a patient that gets upset over the fact that their doctor may not have checked the results in the time frame that the patient thinks is required? Remember doctors have to step out from the connected world here and there to see patients and conduct exams, so we are back to looking for balance here again.
From the website:
“While a number of systems offer a one-way interface to or from an EHR, to date, only one device information system can provide a bidirectional interface. The Paceart System supports universal integration of device data, and the Connected Systems Gateway provides a real-time message-oriented interface that is commonly accepted by EHRs. Paceart HL7 connectivity ensures the seamless flow of cardiac device information to the EHR (Inbound Messages) and of patient scheduling and registration data from the EHR (Outbound Messages) into Paceart.”
The CareLink Programmer can connect to your clinic’s network via an Ethernet cable or a wireless connection.* For programmers used at remote locations that aren’t connected to the clinic’s network, the programmer will store up to 200 follow-up sessions and automatically transfer data to the Paceart System once the network connection is re-established.”
“Use of SessionSync in a wireless configuration requires a Medtronic-supplied 802.11b wireless card and an established 802.11b-compatible wireless network. The network must support WEP wireless security.”
Now let’s get serious and see a list of supported devices – warning there’s quite a few on this list from Medtronic.
SessionSync software supports direct data transfer for about three-quarters of Medtronic’s implantable cardiac devices
What is still lacking here though is the balance for the physicians who are going to be held responsible for all of this and I stated last year that a portal that combines all device that report data would definitely be in order and of course import data to a medical records system so a "Device Portal” that is open source or non vendor specific to gather and aggregate all of this certainly needs to be on the agenda, otherwise the doctors are going to be driven “nuts” as you can only market this so far and claim an area of responsibility as they need to spend time with patients too, you know the old fashion stuff like a conversation on the phone or via the web and personal visits in the office too. Medtronic has aggregated their information into a portal, but what about everybody else and their devices? I can’t help but think these are some of the issues that cause people who work at the FDA to lose sleep at night too.
Wireless Healthcare Medical Devices and the FDA – The Reasons They Are Slow to Come to Terms
If you don’t want to do cellular as a patient, then we have the “Home Monitoring Device” that plugs in that can work without either a land or cellular line.
Having this capability is great but we need to manage it as well so it does not turn out to be too disruptive and too big of a distraction for all concerned. I like technology and all it can do and writing this post even made me tired and it took a while to research all of this to hopefully bring this into layman’s terms where hopefully readers can get some idea as to what is going on out there. Maybe we should hook up Congress to some Home Monitors while they are in the process of making laws so they can become participants and see what is happening outside the walls they live within Washington DC? I think more would literally fall over with shock to see some of this and it’s use.
Let’s not forget privacy here too, and how much of this information will go to the insurers at some point to enable their “scoring” algorithms so they can decide and determine if we are worth insuring or worth paying our claims or not. This is still a very gray area and very few laws here are in place and it’s hard to enforce and create such laws without some digital and algorithmic formulas filed along with the text, patents, and whatever else they want to throw in there. Of course we still need a recall system as that will happen and I suggest this:
Microsoft Tags on CBS Early Show – Wake Up FDA, Pharma and Medical Device Companies –Scan Those Drugs, Medical Devices and Synchronize with an FDA Tag Data Base – Recalls, Theft Tracking and More….
Those tags can go right into the health records and even facilitate the movement of data. BD
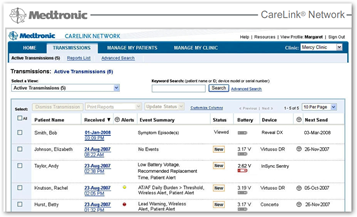
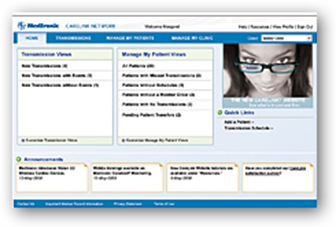
MINNEAPOLIS, May 10, 2010 (BUSINESS WIRE) -- Medtronic, Inc. announced today that it now offers the new Medtronic M-Link(TM) cellular accessory, which provides cardiac device patients with an option to securely send information stored in their implanted devices to their clinics via the CareLink(R) Network using cellular signals, rather than a telephone landline. This simplified connection to the CareLink(R) Network enables clinicians to remotely monitor more patients who are implanted with cardiac devices.
"The M-Link cellular accessory is making remote monitoring accessible to a greater number of eligible patients," said Pat Mackin, president of the Cardiac Rhythm Disease Management business and senior vice president at Medtronic. "Now patients without a telephone landline have a convenient option to access the CareLink Network and take advantage of the benefits that come with remote monitoring, including fewer in-clinic visits and peace of mind from knowing their device data can be transmitted using cellular technology, without the need for a landline."
The M-Link cellular accessory provides patients with a convenient option to stay connected with their clinic from home, work, or while traveling globally. The M-Link cellular accessory securely connects to any CareLink Patient Monitor and allows patients to transmit data from their implanted device directly to their clinic through the secure CareLink Network. It also allows Medtronic CareAlert(R) Notifications to be transmitted when any of the programmable alert conditions from a patient's implanted device has occurred. Physicians and nurses can view the transmitted data through a secure Web site, giving them the opportunity for a "real-time" look at how the patient's device is functioning. The information transmitted is comparable to that provided during an in-clinic device follow-up visit.
Medtronic Launches New Cellular Accessory for the Medtronic CareLink(R) Network - MarketWatch




No comments:
Post a Comment