The FDA is asking for additional trials to be completed before final approval to confirm the results. Trials for this type of drug are not as long as one  would be for a long acting drug, so perhaps we might see this one out there soon.
would be for a long acting drug, so perhaps we might see this one out there soon.
Just this week there was an FDA submittal for inhalable insulin too, so it appears to be a new route for taking medication. The only thing missing is a Bluetooth connection as I posted for another new inhaler for asthma. MAP also has an asthma inhaler in clinical trials so I guess we will have to wait and see if they meet up with the folks at Cambridge Consultants. BD
Mannkind Submits Afresa –Inhaled Insulin Device/Drug to the FDA
MAP Pharmaceuticals’ experimental orally inhaled migraine drug reached all four goals of a late-stage clinical study, the company said Tuesday. Its shares more than doubled.
The drug, Levadex, showed statistical significance at two hours compared with a placebo at relieving common symptoms of migraine: pain, nausea, and sensitivity to light and sound.
Levadex also reached secondary measures of the 792-patient trial, including pain relief at 30 minutes and sustained relief for 48 hours.
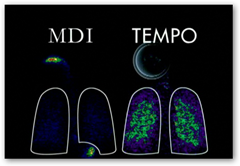
This is how it compares to the old style MDI inhalers with more drug delivered to the lungs.
It is full of electronics for sure, room for a chip in there too I’m sure.
MAP Shares Soar a s Migraine Drug Advances - NYTimes.com
Related Reading:





0 comments :
Post a Comment