This is great news as it can be used for as little as 2 or 3 days and be effective as you don’t really know which AK will turn into cancer or not. In clinical trials more 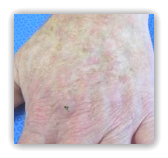 treated with the gel were cleared from the actinic keratosis than those with a placebo. BD
treated with the gel were cleared from the actinic keratosis than those with a placebo. BD
FDA approved ingenol mebutate (Picato, LEO Pharma) gel (0.015%, 0.05%) for the topical treatment of actinic keratosis (AK). It is the first topical AK therapy that can be used for as few as 2 or 3 consecutive days.
AK is a precancerous condition caused by cumulative sun exposure that has the potential to progress to squamous cell carcinoma (SCC), the second most common type of skin cancer. Picato 0.015% gel is used once daily on the face and scalp for 3 consecutive days; Picato 0.05% gel is used once daily on the trunk and extremities for 2 consecutive days.
“The approval of Picato gel is very exciting as it will give physicians and health plans an effective 2- or 3-day topical solution for actinic keratosis, which can lead to squamous cell carcinoma,” John Koconis, president and CEO of LEO Pharma, told Formulary.
According to the American Academy of Dermatology (AAD), 1 in 5 Americans will develop skin cancer in the course of their lifetime. Studies show that about 65% of SCCs begin as untreated AK, and guidelines from the AAD estimate that 60% of predisposed persons older than age 40 years have at least 1 actinic keratosis.



0 comments :
Post a Comment