As mentioned the device and the $40 kit that goes along with the app sold on the App Store was featured at a TED Long Beach Convention earlier this year. The FDA wants a chance to discuss and Biosense has stated they will work with the FDA and at the link below I thought it would be one that the FDA would question as well and here we have it.
“This might be one of those apps if used in the US for the FDA to take a look at for a stamp of approval”
New Mobile App Does Urinalysis With the IPhone–Lab on a Phone
UChek works with test strips made by Siemens and Bayer which do not have approval to be used in an automated algorithmic process. As I reported earlier the device is being tested at a hospital in India. The app can be downloaded but needs the $40 device to work with it. 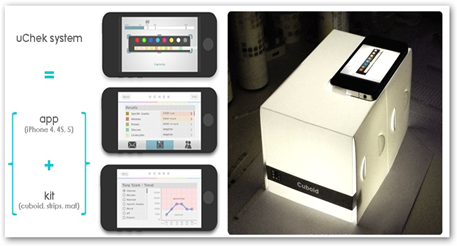
Now that the FDA has stepped in it could be a while before the Android app is released. As mentioned, this is the first letter of it’s kind sent from the FDA and it will be interesting to see if more are forthcoming as they do have their eyes open with watching what kind of mHealth apps are being developed and sold. BD
An iPhone application that lets users check levels of blood, protein and other substances in their urine is the first target of U.S. regulators seeking boundaries in a burgeoning industry for medical diagnosis on-the-go.
UChek works with test strips made by Siemens AG (SIE) and Bayer AG (BAYN), which are only approved for visual reading and require new clearance for automated analysis, the FDA said in the letter. The agency has said it wants 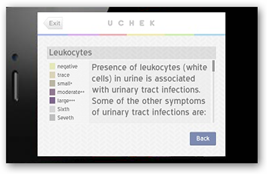 stricter rules for apps that directly diagnose or treat conditions, proposing in 2011 to apply similar quality standards as for heart stents, ultrasound machines and other medical devices.
stricter rules for apps that directly diagnose or treat conditions, proposing in 2011 to apply similar quality standards as for heart stents, ultrasound machines and other medical devices.
The app needed to run the $40 automated system became available in Apple Inc. (AAPL)’s App Store earlier this year after being touted at the technology conference TED2013 at the end of February in California. The FDA told Biosense the company may need to gain agency clearance for the entire system, including the strips. Depending on how a company responds, the FDA may follow up with a warning letter that sets out specific violations of the law that must be addressed immediately, Rivers said.



0 comments :
Post a Comment