Big decision...and who pays for the repair and removal too? and what about those that have dual implants, one for defibrillating and one for being a pacemaker? Tough questions both for patients and the physicians...BD
MORRISTOWN, N.J. — For one heart patient, there will be no more agonizing over whether the implanted device meant to save his life might kill him instead.
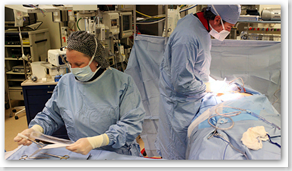
On Tuesday, two months after Medtronic warned doctors and nearly a quarter-million patients that a wire, or lead, connecting their electronic defibrillators to their hearts might break, the 48-year-old patient had the lead and defibrillator removed and replaced. It required a painstaking 90-minute operation here at Morristown Memorial Hospital.
“This has been a nightmare,” said the Morristown patient. He described his fears and agreed to let a reporter observe the procedure on the condition that his identity remained private. Most Fidelis users will not end up needing such an operation, although all have been advised to check with their doctors. But the number of patients with the potentially faulty leads has made this the most widespread problem yet involving a heart device. And the episode has drawn renewed scrutiny to the way medical devices — particularly heart leads — are approved and regulated in this country. The episode has led to investigations in both houses of Congress. One reason for Wall Street’s relief is the widespread agreement among doctors that most patients whose Fidelis leads have not already fractured are better off simply leaving them in place. The caveat is that the defibrillators need to be reprogrammed and monitored, to improve the odds of catching any developing fractures early.



0 comments :
Post a Comment