Used for the detection of Sepsis in the ER....I recently posted an article about Vanderbilt University's technology to facilitate defining the symptom as quickly as possible...technology on the move with data systems to handle....BD
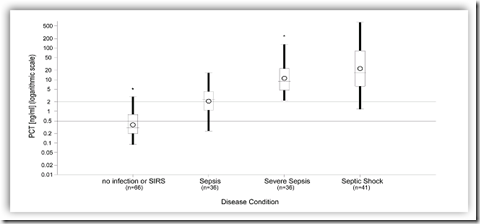
BRAHMS USA, Inc announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its patented Procalcitonin (PCT) Kryptor® test. The automated test will be used in critically ill patients on the first day of ICU admission as an aid to assess their risk for progression to severe sepsis and septic shock.
Sepsis is a complex clinical syndrome that is defined as the systemic inflammatory response of the body to an infection. It is the most common underlying cause of death in non-coronary ICUs where the mortality rate can be as high as 32 percent or 54 percent in cases of severe sepsis or septic shock, respectively. Early recognition of sepsis and timely initiation of appropriate therapy is key for survival from this potentially devastating condition.
BRAHMS Receives FDA Clearance To Market Automated Procalcitonin (PCT) Test



0 comments :
Post a Comment