This is part of the FDA’s efforts for transparency and to improve communications. The site included “basic” information to help companies communicate with the agency. The commitment is to release an answer in 5 days for inquires.
The recall area still sucks and they need to get a system in place, like this: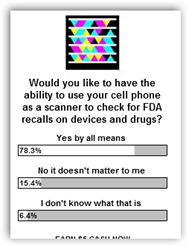
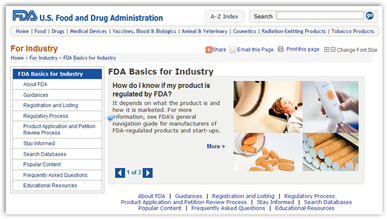
 The FDA has introduced a new Web resource called FDA Basics for Industry to help companies and others save time and resources in their interactions with the agency. The website includes basic information about the regulatory process, including information that is frequently requested by industry.
The FDA has introduced a new Web resource called FDA Basics for Industry to help companies and others save time and resources in their interactions with the agency. The website includes basic information about the regulatory process, including information that is frequently requested by industry.
Survey says we want bar codes on drugs, devices and over the counter products! BD
Abbott Diabetes Care Recalls Tons of Glucose Test Strips–The US Has the FDA Recall Blues-Solution Has Been Touted Here for a Year-It’s Time for a Fix–Readers Have Voted
As part of the agency’s ongoing transparency initiative, the site is one of the 19 action items contained in a 46-page report titled FDA Transparency Initiative: Improving Transparency to Regulated Industry. Other action items include setting an agency-wide expectation that e-mail questions to the FDA involving the regulatory process will, whenever possible, receive an answer within five business days, or a response stating when an answer can be expected; making agency presentations at key meetings widely available; and developing and executing a project to promote uniform processes and procedures across field districts



0 comments :
Post a Comment