If you read the third paragraph here it sounds like the FDA was not satisfied with the device overheating and stated the images were useless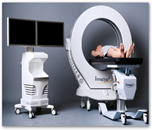 in 2010. If you visit the website the company filed for Chapter 11 in May of this year. The SEC is looking for financial penalties and wants to bar the CEO from serving an an officer of a public company. The product in question was a 3-D dimensional scanner. Perhaps if they work on the technology it could at some point be perfected but the SEC due to the false FDA statements does not feel like it should be under the leadership of the current CEO. BD
in 2010. If you visit the website the company filed for Chapter 11 in May of this year. The SEC is looking for financial penalties and wants to bar the CEO from serving an an officer of a public company. The product in question was a 3-D dimensional scanner. Perhaps if they work on the technology it could at some point be perfected but the SEC due to the false FDA statements does not feel like it should be under the leadership of the current CEO. BD
The Securities and Exchange Commission accused a Burbank medical imaging device company and its chief executive of fraud for allegedly misleading investors about the Food and Drug Administration’s views of one of its products.
In a lawsuit filed Tuesday in federal court in Los Angeles, the SEC said Imaging3 Inc. and its founder and chief executive, Dean Janes, made false statements about the company’s chances of gaining FDA approval of a three-dimensional scanner used in medical diagnosis.
After the FDA denied approval of the device in 2010, Janes told investors that the FDA’s issues were “not substantive” and largely “administrative,” the SEC said. Janes failed to mention that in an October 2010 letter, the FDA said the device had a potential for overheating and that sample images appeared “scientifically invalid and useless.”



0 comments :
Post a Comment