The woman lost her arm and this case has been in the news quite a bit lately and was somewhat complicated and boiled down to warnings and how the drug was introduced into the patient’s vein and hit an artery instead causing her to have complications and her arm amputated. It almost makes one want to run to the internet first and see how IV drugs are supposed to be administered.
As far as the vein portion and hitting the target, there is technology now called the Vein Viewer that can help that situation and it is a fascinating 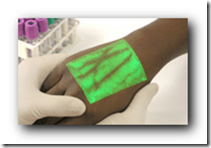 piece of equipment, something every oncologist would love to afford in the office for chemotherapy too I would guess. As a patient, I wouldn’t mind seeing one of these in every medical office for that matter. BD
piece of equipment, something every oncologist would love to afford in the office for chemotherapy too I would guess. As a patient, I wouldn’t mind seeing one of these in every medical office for that matter. BD
Luminetx Infrared VeinViewer prevents a few less “sticks”
We all hate that feeling of getting stuck by the needle for a shot, or inserting an IV. The vein viewer can help take the guess work out of multiple sticks. I posted about this device last year and the small article has had so many views it was time for an update.
I can’t think of anyone who would not like or welcome this device by all means. If one were a patient that required regular injections or IV set ups, this could be a real blessing, not only for the patient, but also for the clinician drawing blood and make their job a lot easier with a lot less apologies for the “dead sticks” and having to locate the vein all over. The device is also being used for the treatment of varicose veins.
WASHINGTON — In one of the most important business cases in years, the Supreme Court on Wednesday ruled that a drug company is not protected from injury claims in state court merely because the federal government had approved the product and its labeling.
The key issue before the justices was whether the Food and Drug Administration’s approval of drug labels should pre-empt lawsuits in state courts contending, as Ms. Levine’s did, that the labels did not contain adequate warnings.
Wyeth’s lawyers, supported by the Bush administration, had argued that the company provided “ample, lavish warnings,” as one attorney put it, and that Wyeth should not have been held liable, because the Food and Drug Administration had approved the label on the drug in question, Phenergan.
If Phenergan is exposed to arterial blood, it causes swift and irreversible gangrene. Therefore, it is typically administered by intramuscular injection. Ms. Levine’s lawyers said an intravenous drip is also quite safe.
But a physician’s assistant used a third method, injecting the drug into what she thought was a vein, using a technique known as “IV push.” The assistant apparently missed a vein and hit an artery instead, causing Ms. Levine’s right hand and forearm to turn purple and black in the following weeks, leading to amputation of much of her arm.
Drug Approval Is Not a Shield From Lawsuits, Justices Rule - NYTimes.com



0 comments :
Post a Comment