The Mammosite procedure has been approved for a few years and now the improved process with multi-lumen design has been approved by the  FDA. So far, procedures have been provided with a single lumen device. The website has a lot of information and a video to explain further how it works. The difference with the Mammosite and other seeding procedures is that the “seed” does not stay inside your body, as which is done with prostate cancer procedures. Brachytherapy is widely used to treat prostate cancer because it allows high doses of radiation to be directed to a small area. Accuracy is important and in the news of late reference to prostate treatment we had the VA and a few of their doctors questioned as some of the seeds missed their targets, in other words were not placed properly and caused damage with radiation exposure in other areas.
FDA. So far, procedures have been provided with a single lumen device. The website has a lot of information and a video to explain further how it works. The difference with the Mammosite and other seeding procedures is that the “seed” does not stay inside your body, as which is done with prostate cancer procedures. Brachytherapy is widely used to treat prostate cancer because it allows high doses of radiation to be directed to a small area. Accuracy is important and in the news of late reference to prostate treatment we had the VA and a few of their doctors questioned as some of the seeds missed their targets, in other words were not placed properly and caused damage with radiation exposure in other areas.
The Mammosite has the “balloon” which is inserted after the removal of a tumor or lump and is placed in the cavity where the tumor had been located prior to removal. I had originally posted about the Mammosite procedure a few months ago.
Breast Cancer Treatments – What is a MammoSite?
The procedure is complete in 5 days. With the current closure of nuclear reactors, isotopes may delay treatment, depending on availability. BD
“When used as primary radiation therapy, 2 treatments are administered per day, for up to 5 days, to deliver the prescribed radiation dose (typically 34 Gy). When used as a boost with external beam radiation, a typical prescription requires treatment for 1-2 days.”
There is also a lab now that can test for “cancer of the unknown” with breast biopsies.
Genomics lab Opens in Huntington Beach – Cancer of the Unknown Primary with Breast Cancer
BEDFORD, Mass., Sept. 1 /PRNewswire-FirstCall/ -- Hologic, Inc. (Hologic or the Company) (Nasdaq: HOLX), a leading developer, 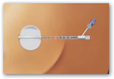 manufacturer and supplier of premium diagnostics, medical imaging systems and surgical products dedicated to serving the healthcare needs of women, today announced the U.S. Food and Drug Administration (FDA) cleared the Company's 510K application for the MammoSite((R)) ML radiation therapy system. With its multi-lumen design, this new device gives radiation oncologists the ability to shape the radiation dose for typical cases and treat patients who are otherwise not appropriate candidates for traditional brachytherapy. Hologic's MammoSite therapy system, first cleared by the FDA in 2002 as a single-lumen device, is the most widely used form of accelerated partial breast irradiation (APBI) in the United States. It has been used to treat more than 50,000 breast cancer patients in the U.S.
manufacturer and supplier of premium diagnostics, medical imaging systems and surgical products dedicated to serving the healthcare needs of women, today announced the U.S. Food and Drug Administration (FDA) cleared the Company's 510K application for the MammoSite((R)) ML radiation therapy system. With its multi-lumen design, this new device gives radiation oncologists the ability to shape the radiation dose for typical cases and treat patients who are otherwise not appropriate candidates for traditional brachytherapy. Hologic's MammoSite therapy system, first cleared by the FDA in 2002 as a single-lumen device, is the most widely used form of accelerated partial breast irradiation (APBI) in the United States. It has been used to treat more than 50,000 breast cancer patients in the U.S.
The MammoSite systems are comprised of an inflatable balloon catheter in which a radioactive source is introduced for therapy delivery. The inflatable balloon is inserted into the surgical cavity remaining after removal of the tumor. This local placement of the balloon provides for therapeutic delivery of a five-day course of radiation to the tissue most likely to contain residual cancerous cells following surgery, while reducing radiation exposure to adjacent healthy tissue. Using the MammoSite multi-lumen catheter, the radiation oncologist has the ability to shift the radiation dose to the areas that need it most and shift the dose away from areas that do not require it.
FDA Clears Hologic's MammoSite(R) ML Radiation Therapy System for the Treatment of... | Reuters
Related Reading:





0 comments :
Post a Comment