The software is what stores, creates and overall handles the designed treatment programs for patients. This is good that the NIH wants some audit trails to track dosages and even expand the information to be contained in EHRs and PHRs. I would think an internal program at a hospital to run a tolerance query against standards couldn’t hurt either. When I was writing software, audit tables we a life saver in quite a few areas and I had screens that ran reports to create reports. A person would not have time to run these reports and this is something that would need automation through SQL server or another equivalent program/data base. 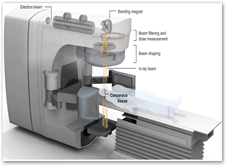
NIH Getting Involved in Radiation Overdoses – Planned Requirement for a Audit Trail to Track Patient Radiation Doses and Eventually Add to EHRs as well as PHRs, Google Health and HealthVault
If you read this feature in the New York Times it was all software related. When the development time for automated processes is needed as well as the software that runs the settings on the MRI or CT scanner, don’t push and rush the programmers. Code writers are sometimes our own worst enemies in the fact that we make it look so simple by the time the entire modules all run, but behind the scenes there’s a lot of work and a lot of debugging that takes place before any of it sees the light of day. The average person is not usually ever exposed to the work behind the scenes and thus thinks that some of these chores can be written quickly, not always the case. BD
Radiation Procedures Gone Sour – Software and Other Related Failures Lead to Death and Lifetime Illnesses
WASHINGTON -- The U.S. Food and Drug Administration (FDA) says it wants to issue new safety requirements for manufactures of computed tomography (CT) and fluoroscopic devices to reduce unnecessary radiation from medical imaging.
The FDA's plan focuses on three procedures with high radiation doses: CT, nuclear medicine studies, and fluoroscopy. These are the greatest contributors to total radiation exposure within the United States population, the FDA said. That's because they require much higher radiation doses than other radiographic procedures, such as standard X-rays, dental X-rays, and mammography.
"The amount of radiation Americans are exposed to from medical imaging has dramatically increased over the past 20 years," Dr. Jeffrey Shuren, director of the FDA's Center for Devices and Radiological Health, said in a prepared statement. "The goal of FDA's initiative is to support the benefits associated with medical imaging while minimizing the risks."
FDA Wants New Radiation Safety Standards for Popular X-Rays - ABC News



0 comments :
Post a Comment