That’s the answer here as to manually collect and analyze with today’s standards and availability of information is almost not possible. This also goes to show that Health IT and the FDA are in fact emerging areas to where one is going to need to work closely with the other. The link below will give you an idea as to some of the devices that are emerging to collect data. Check it out if you want to see what’s out there today, no longer Star Wars.
Wireless Monitoring With Medical Devices – There are Many Posts About These at the Medical Quack
You could in fact in the future be wearing a shirt like this one below to participate in a trial for medical devices that collects all kinds of data as trials are one of their market areas.
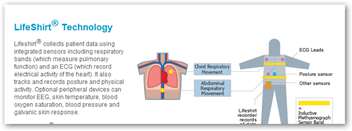
As a matter of fact, to participate in a trial for one device, you may be outfitted with one or two other devices that report the data needed by the FDA for safety and accountability as well as the success rate of the medical device. More information and the need for data drives more audit trails and software development for both medical devices and drugs. BD
SILVER SPRING, Md., March 31 /PRNewswire-USNewswire/ -- The U.S. Food and Drug Administration announced today that it will begin implementing a requirement that device manufacturers provide readily available information in certain premarket applications on pediatric patients who suffer from the disease or condition that the device is intended to treat, diagnose, or cure, even if the device is intended for adult use.
Very few devices are developed or assessed specifically for use in pediatric patients, those 21 or younger at the time of treatment or diagnosis. This effort will provide a better understanding of which devices developed for use in adults should be assessed or modified for use in pediatric populations. The requirements, contained in the Food and Drug Administration Amendments Act of 2007, will also improve the agency's ability to track the number of approved devices for which there is a pediatric subpopulation who could benefit and the number of approved devices labeled for use in pediatric patients.



0 comments :
Post a Comment