In women’s health, this could certainly be viewed as a plus and an alternative for an immediate hysterectomy with the procedure and device that can be done in a doctor’s office. If you are a female, you know that this is a very common condition that gets a lot of talk. 
The device already carries the European CE approval. As with most devices today software plays an important role and this has been part of the upgrade. BD
From the website:
The HTA System is designed to offer:
- A therapy that fully conforms to uterine lining by circulating heated saline
- A gravity flow system which is designed to:
- Prevent fluid escape through the fallopian tubes
- Treatment of a partial septate uterus
- Therapy in the presence of intramural uterine fibroids (≤4 cm)
- Hysteroscopic visualization which promotes proper device placement throughout treatment cycle
Boston Scientific is committed to continually improving patient care through innovation. The HTA System now includes a disposable heater canister, software 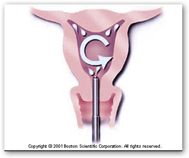 upgrades, and the HTA ProCerva Sheath with the cervical seal assist and tenaculum stabilizer.
upgrades, and the HTA ProCerva Sheath with the cervical seal assist and tenaculum stabilizer.
NATICK, Mass., May 18 -- Boston Scientific Corporation (NYSE: BSX) today announced U.S. Food and Drug Administration (FDA) approval of its Genesys HTA™ System for the treatment of menorrhagia. The Genesys HTA System is a next-generation endometrial ablation system designed to ablate the endometrial lining of the uterus in premenopausal women with menorrhagia.
Menorrhagia is abnormally heavy and prolonged menstrual bleeding. An estimated 10 million women in the U.S. suffer from this condition.
The Genesys HTA System represents a significant advance in Boston Scientific's endometrial ablation technology. It features a smaller and lighter console, simplified set-up requirements, and an enhanced graphic user interface which offers step-by-step guidance through the procedure. The System also incorporates several technology upgrades designed to improve operating performance while delivering the same proven clinical therapy of the Company's current HTA Endometrial Ablation System
FDA Approves Boston Scientific's Genesys HTA™ System - May 18, 2010



You made some good points there. I did a search on the topic and found most people will agree with your blog.
ReplyDelete