This heated debate has been kicking around for a while now and until retail pharmacies actually pushed the button with making test available for purchase of the “box” at the stores things were somewhat moving along ok for those who wanted to have their DNA sequenced. Today, things get very confused when we as consumers try to differentiate the difference between knowledge and marketing, in other words do we need/want this, or is it just one more attempt to make a buck. I have been following this along and the strategic alliances that were forming here were a bit disturbing with some of what we were seeing out there, so perhaps the FDA stepping in here was not a bad thing. You can read a couple paragraphs from a recent prior post below and catch the drift.
“box” at the stores things were somewhat moving along ok for those who wanted to have their DNA sequenced. Today, things get very confused when we as consumers try to differentiate the difference between knowledge and marketing, in other words do we need/want this, or is it just one more attempt to make a buck. I have been following this along and the strategic alliances that were forming here were a bit disturbing with some of what we were seeing out there, so perhaps the FDA stepping in here was not a bad thing. You can read a couple paragraphs from a recent prior post below and catch the drift.
Pharmacy Benefit Managers Striking Alliances with Genomic Test Companies – A New Left Hook in Shaping Portions of Healthcare Reform With Genetic Benefit Managers?
“Insurance companies now are taking a look at the cost side and are exploring some of these areas with the pharmacy benefit managers. The insurance companies can only go so far with the GINA laws and their new partners for information appear to be in the area of the PBM; however, how all this works is continuing to develop. The pharmacy benefit managers more than likely will be playing a larger role with the physicians with information about prescribing drugs for patients, again based on genomics and possibly recommending that certain patients be given certain tests. It gets a bit complicated here as the payers are still in their own rite, trying to figure out which tests they should allow, and again this is based on cost. Getting a prescription for some simple allergy relief one day might become a very complicated issue with having a multitude of data to look at before that prescription is written.
Next up, privacy with sharing patient information with payers and how this will get utilized too, as a payer will need to know, or want to know that a DNA test shows before potentially allowing some medications and treatments to be covered, especially when it comes to areas like oncology for example where the cost is high. CVS has affiliated itself and through the Minute Clinics has an arm to provide DNA testing within the stores/clinics. You may have a provider wanting to prescribe a certain medication and a negative given for coverage based on a DNA test on file, just some new potential conflicts that could be on the horizon as interpretations are queried.”
I wrote about the FDA decision below.
FDA States Genetic Tests Are Considered a Medical Device And Thus Need FDA Approval
There are also some hospitals and universities that are working to offer genomic education to physicians which is a good thing.
FDA Listing of In Vitro Diagnostics - Letters Sent to 5 Companies
Navigenics has taken the approach to include the physician in this area and that is a good thing so if patients have had some sequencing done, they have an idea as to what is going on.
Beth Israel Deaconess Medical Center and Navigenics Partner to Make Personalized Genomics Training Available for Physicians
If you take a look at the companies receiving letters from the FDA, it also included Knome, founded in part by George Church, which is where gene sequencing all began at Harvard. I’m sure the company along with 23AndMe will answer the FDA inquiries and we will hear the outcome. These companies have not made any retail affiliations and are not on the “retail cash cow” to make profits from consumers in this fashion, again that is what blew the doors off the barn here with marketing and actual consumer/patient/doctor information sources being exploited. I still feel that an individual with proper guidance should be able to have their genes sequenced, but the flurry created for profits with retail operations and control over medications by pharmacy benefit management lead this to get out of hand.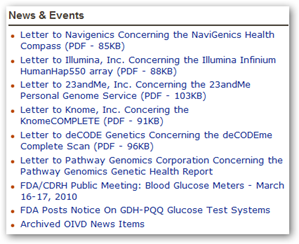
This is no way is related to having a specific genomic test done for one or two medications for a selection of a drug to be used for treatment. That part of the clinical side is already inter woven into treatment plans and is done in coordination with a physician requesting a test. The danger of the retail involvement here comes around to some privacy issues as Pharmacy Benefit Management data bases DO NOT fall under HIPAA, thus who knows what information would be bought and sold about you with reference to “wellness groups” and especially those owned by health insurance companies. Read that again if you missed it, wellness companies owned by health insurance companies to drive profits to the bottom line, and if you happen to get healthier in the profit making process then they can sell this as a real success to investors and others.
Granted there’s a lot of good to wellness programs if they are implemented properly and work as a “true”partner to the consumer with information, but trying to figure out which is which sometimes is a problem with the vast marketing and intelligence added to what is seen on the web today. I know as a consumer I want a “real”partner and not a marketed fabrication to just drop money to the bottom line profits, I feel that is a better selection process. Basically this ruling from the FDA results bottom line from health insurance companies wanting some bottom line profits and control instead of working from a pure motivation angle. It is what it is and if you read between the lines, and there are many posts on this blog that address different areas, you will see how they are inter twining themselves into so many other areas outside of providing care, some good and some not so good, but pay attention to these alliances as you will need to really learn how to read between the “marketing” lines today.
some bottom line profits and control instead of working from a pure motivation angle. It is what it is and if you read between the lines, and there are many posts on this blog that address different areas, you will see how they are inter twining themselves into so many other areas outside of providing care, some good and some not so good, but pay attention to these alliances as you will need to really learn how to read between the “marketing” lines today.
On the web I read where Pathway Genomics is backing out of the consumer marketing side of sequencing who was the partner for Walgreens and one of the recipients of the FDA letters.
Walgreens Stores To Offer Genetic Testing From Pathway Genomics Over the Counter
Pharmacy benefit managers like Medco will be looking at their future plans of action too since they purchased a partner who does genomic sequencing earlier this year called DNA Direct and I was not able to locate who their “sequencing machine” partner is at present.
Medco Also Entering Into the Field of “Genetic Benefit Management” With Making New Tests Available
With the retail profit cash cow going into actions, this certainly appears to have caught the attention of the FDA and it will be interesting to see how it goes from there. In a good way, the FDA did clamp down on the “marketing for profit areas here” and hopefully their action will help keep genomics clearly in focus with clinical outcomes and studies without furthering the interest in the over all retail cash cow and genetic benefit management factors being marketed loosely, without a full addressing of privacy issues at hand. BD
The U.S. Food and Drug Administration has decided that the personalized DNA tests sold by companies like 23andMe are medical devices subject to government regulation, and the federal agency says the tests must be vetted by regulators if they are to remain on the market.
That’s what Navigenics intends to do, according to this post on the company’s blog:
We believe our services comply with all existing federal and state regulations, and look forward to continuing our dialogue with the FDA to ensure transparency and the optimal use of genetic information as it applies to personalized medicine.
23andMe took a similar position, arguing that people have a right to know their genetic information and that the FDA should not stand in their way.



0 comments :
Post a Comment