You can almost bet that inhalers such as this one, when they do get replaced will have some type of wireless capability. That is the way drug delivery is going so  patient compliance can be measured, so look for more of that type of delivery to show up soon. All big pharma companies have technology partners who develop such devices for drug delivery. One in particular that I have talked about here is Cambridge Consultants, who does make one of those wireless, audit trail, compliance tracking devices. The link below is worth a watch as your inhaler of the future might become very social, if you will with wireless connections and data trails for compliance. Yup, let’s send that compliance information to the insurance company so they can deny your claim if you are a couple hours out of compliance and that is the way this is headed, trust me with everyone and their business intelligence systems on steroids today.
patient compliance can be measured, so look for more of that type of delivery to show up soon. All big pharma companies have technology partners who develop such devices for drug delivery. One in particular that I have talked about here is Cambridge Consultants, who does make one of those wireless, audit trail, compliance tracking devices. The link below is worth a watch as your inhaler of the future might become very social, if you will with wireless connections and data trails for compliance. Yup, let’s send that compliance information to the insurance company so they can deny your claim if you are a couple hours out of compliance and that is the way this is headed, trust me with everyone and their business intelligence systems on steroids today.
The Minder Wireless Device Connects to Collect Patient Medical Data and Transmit Via Wireless Network to Medical Record Systems Via HL7 Standards
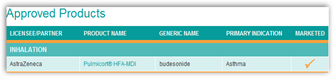
As you can see here pulling the product takes a chunk of revenue from the Astra technology partner, SkyePharma. It would be nice someday to have a generic  inhaler for delivery of drugs as I can see this mounting with who has the best mouse trap here as well as far as inhaled drug delivery systems. As we know, MannKind spent a lot of money on their inhaled delivery system only to get turned down by the FDA, so again how many millions and billions are going to be spent on these delivery systems you might ask?
inhaler for delivery of drugs as I can see this mounting with who has the best mouse trap here as well as far as inhaled drug delivery systems. As we know, MannKind spent a lot of money on their inhaled delivery system only to get turned down by the FDA, so again how many millions and billions are going to be spent on these delivery systems you might ask?
MannKind Corp Waiting for FDA Approval of Inhaled Insulin–Afrezza-An Army of Inhaler Manufacturers Awaits With Products for Consumer Compliance Monitoring
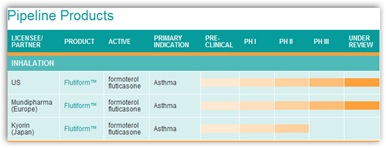
You can see they have more inhalers in the pipeline too so again this field seems to be getting very crowed very quickly.
Inhaler delivery system almost seem like they might be better of being developed via the NIH with a team of non profits on all of this as we don’t need the huge amount of inhalers on the market as that is only worse for both consumers and doctors, and when you have recalls, well another mess. BD
LONDON, March 7 (Reuters) - AstraZeneca (AZN.L) is ending production of the pressurized metered dose inhaler version of its asthma drug Pulmicort, due to technical problems, in a move that will hit its technology partner SkyePharma (SKP.L).
SkyePharma developed the formulation for the medicine and earns a mid-teens percentage royalty on its sales. Royalties from the product comprise about 5 percent of SkyePharma 's revenues.
Shares in SkyePharma tumbled 16.7 percent in early trade on Monday, while AstraZeneca slipped 0.4 percent
Pulmicort pMDI is only sold in some countries and other AstraZeneca products, including Pulmicort Turbuhaler, Pulmicort Respules and Pulmicort Flexhaler are not affected because they use different devices.
UPDATE 2-Astra pulls asthma product, hitting SkyePharma | Reuters



0 comments :
Post a Comment