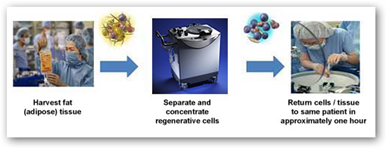
I started covering Cytori back in 2007 and if you do a search you can find plenty here at the Medical Quack to include the initial use in Europe and the fact that GE decided to invest and help commercialize. The system takes a women’s own fat cells and basically recycles them for breast restoration purposes, like a big dishwasher for stem cells for lack of a better way to describe what it does. The big concern from doctors on the use of the system and procedure was the fear that cancer cells would re-produce but from the results of the study it turns out this has not been the case.
GE Healthcare to Commercialize Cytori Therapeutics Stem Cell Technology – Regenerative Medicine
The video below talks and shows a bit more about how it works. In Australia they are doing stem cell trials with a different process to regrow breasts.
In my mind it certainly beats the alternative of implants. They are still in search of obtaining more clinical data as the machine has been around since 2007 so there’s no real long term information out there yet just simply due to the young age of the technology. Japan was the first country to jump on the bandwagon with Cytori. It takes about 6 weeks to re-grow breasts, not bad as you have that same amount of time just about to recover from implant surgery in round terms. Again with using one’s own stem cells, there’s no rejection issues. BD
ZUG, Switzerland (Dow Jones)--A long-term study looking at Cytori Therapeutics Inc's (CYTX) tissue regeneration method--which uses stem cells from a person's own fat to reconstruct breasts--shows the procedure to be safe when used on former breast cancer patients who suffered from scars or had part of their breast removed, the U.S.-based company said Wednesday.
The findings could help thousands of former breast cancer patients benefit from a minimally invasive method to restore breasts disfigured by surgery.
It could also open a potential multi-billion dollar market for Cytori, which generated sales of about $6 million in the first nine months of 2010 and hopes to broaden the use of its method outside the cosmetics market.
Cytori said the fresh 12-month trial of its Celution System, which included 71 patients, confirmed the findings of an earlier six-month trial which registered high physician and patient satisfaction ratios of around 80%. Cytori said the comprehensive data are being prepared for peer-review and should be publicly available later this year.
Study Shows Cytori's Stem Cell Breast Reconstruction Is Safe - WSJ.com





Why is there no urther information more than a year ad a half after this article was published?
ReplyDelete