The Edwards SAPIEN transcatheter heart valve is made of bovine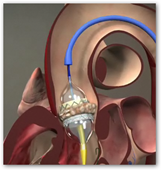 pericardial tissue leaflets hand-sewn onto a metal frame, is implanted via one of two catheter-based methods. The product was recently approved by the FDA for patients who cannot undergo full open heart surgery due to their physical conditions so the device will now open opportunities for more patients who need surgery to have it done. The valve procedure improves quality of life and survival rates with patients who have been implanted with the device compared to those who only received medical treatment. Many patients who were bed ridden now may be able to get out of bed and resume a closer to normal life.
pericardial tissue leaflets hand-sewn onto a metal frame, is implanted via one of two catheter-based methods. The product was recently approved by the FDA for patients who cannot undergo full open heart surgery due to their physical conditions so the device will now open opportunities for more patients who need surgery to have it done. The valve procedure improves quality of life and survival rates with patients who have been implanted with the device compared to those who only received medical treatment. Many patients who were bed ridden now may be able to get out of bed and resume a closer to normal life.
Symptoms of aortic stenosis have several symptoms to include chest pain, shortness of breath and fainting. BD
Newswise — UCLA has performed its first transcatheter aortic
 valve replacement (TAVR), using a new device approved by the U.S. Food and Drug Administration to replace an aortic valve in a patient who was not a candidate for open-heart surgery.
valve replacement (TAVR), using a new device approved by the U.S. Food and Drug Administration to replace an aortic valve in a patient who was not a candidate for open-heart surgery. Ronald Reagan UCLA Medical Center is part of a growing trend of hospitals nationwide offering this new minimally invasive procedure.
Many patients are not good candidates for conventional valve replacement because they suffer from a number of other health issues, and it is estimated that 40 percent of patients do not undergo aortic valve replacement because they are considered inoperable.
The new device is deployed through a catheter — a long tube that is advanced through an artery in the groin up to the heart. Once in place, a balloon at the end of the catheter is inflated, opening the new valve, which starts working instantly.
http://www.newswise.com/articles/view/592664/?sc=rsla&utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+NewswiseLatestNews+%28Newswise%3A+Latest+News%29



0 comments :
Post a Comment