This is a heated topic for sure and when you look at the doctor’s side of this as one MD commented, there’s a lot of counseling and  advising that goes along with the process, especially if the test were to show a positive result. Doctors are already maxed out in so many areas and again this would add for a much longer consult time and the old 99213 consult for a positive result is not going to cut it for the reimbursement time. As mentioned, the FDA has approved the OraQuick test which is expected to sell for around $60 for a consumer to do an over the counter test. One wonders is this the correct route to go to home test first and then go see the doctor? Obviously all who would use a home test would need to be plugged in to a resource to where free initial counseling and advice would be given to get the consumer on the right track to include urging making an appointment to see your doctor.
advising that goes along with the process, especially if the test were to show a positive result. Doctors are already maxed out in so many areas and again this would add for a much longer consult time and the old 99213 consult for a positive result is not going to cut it for the reimbursement time. As mentioned, the FDA has approved the OraQuick test which is expected to sell for around $60 for a consumer to do an over the counter test. One wonders is this the correct route to go to home test first and then go see the doctor? Obviously all who would use a home test would need to be plugged in to a resource to where free initial counseling and advice would be given to get the consumer on the right track to include urging making an appointment to see your doctor.
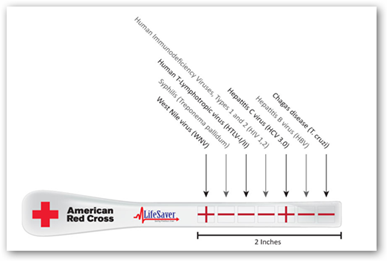
Now let’s look at another aspect of the over the counter test. $60 is not huge, but how many would purchase a product to self test at this price? That answer remains with the consumer of course and with current economic times that could be tough. If you look at the OraQuick test it takes 2 full minutes of swabbing and if not done correctly, the test could be compromised as well. Right now it’s the only thing out there but what if the process were less expensive and easier? I do some consulting work for a company named LifeSaver that is in the process of getting a much simpler test to market and it would cost somewhere around $13 or so for a test and 2 minute results versus 20-40 minutes. Would this help?
If a blood panel is drawn at a doctor’s office then you have to wait as usual for the results to come back from the lab. Corporate labs do a ton of these tests all the time and of late I am hearing about a lot of discrepancies with what is orders and what is returned is not being the same and of course that costs time and money. Better yet, why not work with the blood banks across the US and define a better methodology to screen for HIV, Hepatitis and other diseases? It would save them money as well as some FDA millions of dollars that they get fined as well every year it seems.
FDA Sends Warning Letter to Community Blood Centers of Florida For Not Meeting Guidelines–One Violation States It Takes 87 Days to Notify a Donor They Tested Positive for HIV–Better Solution Here
It appears that the consumer OTC role has been defined with the FDA approval so let’s make it simpler and easier for the consumer. Take a look at LifeSaver Products here and see what you think about a 2 second tongue swipe and results in 2 minutes…and $13 versus $60 for the one HIV test. For a little more cost with the same 2 minute results, multiple tests can be done on one Stik, up to 7 and again even though the price of the Stik goes up it would still be way below blood screenings for each test by far and again, 2 minute results.
If this is in fact the direction we are going, for goodness sakes let’s make the process simple and get the resources lined up for consumers and providers so we have a system we can work with and get accurate screenings done.
LifeSaver’s Saliva-based HIV Stik® Advantages
- LifeSaver has simple, easy-to-use instructions featuring a (+) or (-) sign that virtually anyone can understand.
- LifeSaver has a faster collection rate (seconds vs. 2 full minutes for OraQuick).
- A small amount of saliva is all that is necessary “As easy as licking a stamp”
- LifeSaver has a faster read time (2 minutes vs. OraQuick 20- 40 minutes).
- LifeSaver is $7.00 vs. OraQuick $17.50 to health care professionals.
- LifeSaver guarantees accurate results with a 2 year shelf life.
The LifeSaver Stik® technology will revolutionize the antiquated immunoassay testing methods being used around the world today, and in turn, become a leader in the global healthcare industry. The company still needs a few more investors to get this on the road and you can read more about investor opportunities and distributorships here. When it comes time for public commenting be sure to voice your opinion on how you want a simple process with the necessary resources available on what to do if a positive result is shown. Education is the only way to move this forward and get treatment to those in need. Also take note that many other screenings could be handled with the same technology. BD
(Reuters) - A U.S. health panel may soon make HIV testing as standard a practice as checking cholesterol levels, a move that would fundamentally change how the virus is detected and treated.
The U.S. Preventive Services Task force, a government-backed group of clinicians and scientists, is expected to make a new recommendation on HIV screening available for public comment before the end of the year
Under President Barack Obama's healthcare law, passed in 2010, insurers are required to cover preventive services that are recommended by the task force.
While the task force doesn't factor cost into its considerations, the CDC and other healthcare providers do. Researchers at Stanford University estimate that over a 20-year period, expanding HIV testing to the general U.S. population would reach $27 billion dollars.
The U.S. Food and Drug Administration recently approved the first over-the-counter, self-administered HIV test from OraSure Technologies, which is expected to sell for $60. A positive result would require follow-up at a doctor's office
LeFevre, a primary care doctor in Missouri, cautions that the barriers to testing go beyond the rating of a single agency.
"I can't think of another blood test in all of my practice that carries that baggage," he says of the pre-test consent, counseling, and post-test follow-up that HIV screening requires.
http://www.reuters.com/article/2012/08/19/us-usa-health-hiv-idUSBRE87I04J20120819?feedType=RSS&feedName=healthNews&utm_source=dlvr.it&utm_medium=twitter



0 comments :
Post a Comment