If you have followed my blog then you might remember I had quite a few posts on using bar codes to identify recalls on drugs and devices. 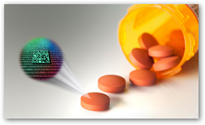 I finally put a bunch of it together on one page and it’s here in my archives. Microsoft recently announced they are out of the bar code business and services will be transitioned to ScanLife. TruTag is another company who has developed the bar coding even further so we can eat or swallow them with no harm, made out of things that are already found in our bodies or in food products we eat.
I finally put a bunch of it together on one page and it’s here in my archives. Microsoft recently announced they are out of the bar code business and services will be transitioned to ScanLife. TruTag is another company who has developed the bar coding even further so we can eat or swallow them with no harm, made out of things that are already found in our bodies or in food products we eat.
This is pretty good that any pill could be scanned for authenticity. How many stories have we read about counterfeit drugs..too many. TruTag is located in Hawaii so developed right in the good old USA too. The silica micro tag is pretty durable and in addition to drugs there’s an open door for use with other products that get copied as well. So far from what I read they have a “specific” reader developed for the process on the drugs and it there’s a phone app for scanning too. I’m sure the FDA if they haven’t already, will be taking a look at this technology as well. On consumer products though, I still wouldn’t mind a bar code for recalls to be available, can’t hurt. Sorry compliance folks this product is not for that:) Someone will try though you can almost bet given a little time here.
The product specifically addresses FDA’s PCID (physical-chemical identifier) guidance for anti-counterfeiting measures. BD
The microtags developed by TruTag Technologies are made from silica — an ingredient already found in many foods, including sweeteners — and are independent of packaging and labels and can be integrated directly into a product’s infrastructure. About the size of a dust particle and smaller than the width of a human hair, the silica “TruTags” provide a
 vast library of unique codes, allowing for improved tracking and authentication of electronics, industrial parts and consumer goods. Each tag’s coded, nano-scale pattern can be scanned using the company’s proprietary instruments. These patterns are like ID numbers that can be associated with a variety of information, such as product strength, site of manufacture, expiration date and country of authorized sale.
vast library of unique codes, allowing for improved tracking and authentication of electronics, industrial parts and consumer goods. Each tag’s coded, nano-scale pattern can be scanned using the company’s proprietary instruments. These patterns are like ID numbers that can be associated with a variety of information, such as product strength, site of manufacture, expiration date and country of authorized sale. Putting product intelligence on a pill, instead of the package, could help drug manufactures and product security teams authenticate their product. A few years ago, GSK, one of the top pharmaceutical companies in the world, was found guilty of felony distribution of adulterated drugs and paid a $750 million fine for mixing up an anti-anxiety drug and a diabetes drug in the same GSK packaging. Had the drugs been equipped with TruTag microtags, the inspectors could have determined where each pill was made to help diagnose where the problem was in their global supply chain.
TruTag has been named a 2014 Technology Pioneer by the World Economic Forum, an award given to 36 companies that are designing, developing and deploying innovative technologies that affect business and society.



0 comments :
Post a Comment