I have added information about a couple of devices that have FDA approval to use transcranial magnetic stimulation in the recent past. We are coming of age to where maybe some of the medications used for depression can be “zapped” away. The study in this article shows some relief with treating depression.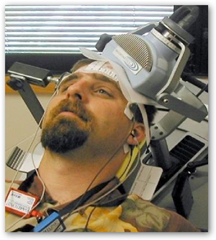 I do have to say just looking at the picture somewhat makes me feel a little squeezy.
I do have to say just looking at the picture somewhat makes me feel a little squeezy.
Brainsway gets FDA nod for device trial – Another Device for Transcranial Magnetic Stimulation
FDA Clears Neurostar® TMS Therapy For The Treatment Of Depression
The entire process per treatment is 37 minutes, so it is something one can squeeze in on the lunch hour if needed. There’s also the more radical treatments for more radical conditions: magnetic seizure therapy is a more robust version of TMS which produces a seizure on purpose and thus requires anesthesia and a similar setting.
The treatment for depression is of course a lighter process and now that clinical trials are underway they are looking for the ability to target other portions of the brain. Overall so far the jury is still out on this one as some have been helped while others were not. The initial time frame was for 3 weeks and those who felt their moods lift were given additional 3 weeks of treatment. The testing is being conducted with individuals who had not responded to the normal medications given for depression. The ones who had success stated their depression remained in remission for several months. Boy this is bold new territory here and I’m sure we will be hearing more as the trials continue. BD
ScienceDaily (May 3, 2010) — Some depressed patients who don't respond to or tolerate antidepressant medications may benefit from a non-invasive treatment that stimulates the brain with a pulsing electromagnet, a study suggests. This first industry-independent, multi-site, randomized, tightly controlled trial of repetitive transcranial magnetic stimulation (rTMS) found that it produced significant antidepressant effects in a subgroup of patients, with few side effects.
Active rTMS treatment accounted for remissions in 14 percent of antidepressant-resistant patients actively treated, compared to about 5 percent for a simulated treatment.
"Although rTMS treatment has not yet lived up to early hopes that it might replace more invasive therapies, this study suggests that the treatment may be effective in at least some treatment-resistant patients," said Thomas R. Insel, M.D., director of the National Institute of Mental Health (NIMH), part of the National Institutes of Health, which funded the study.
"This study should help settle the debate about whether rTMS works for depression," said George, who led the research team. "We can now follow up clues suggesting ways to improve its effectiveness, and hopefully further develop a potential new class of stimulation treatments for other brain disorders."
The treatment aims to jump-start underactive mood-regulating circuitry by targeting the top left front part of the brain with an electromagnetic coil that emits 3,000 pulses over a 37-minute session. It can be safely administered in a doctor's office with few side effects -- unlike more invasive brain stimulation treatments, such as electroconvulsive therapy.
Since the rTMS treatment did not trigger any seizures or notable side effects, the researchers propose that higher levels of magnetic stimulation be used in future studies, as evidence suggests antidepressant effects of such stimulation are dose-dependent. Higher remission rates might also be attainable if rTMS were combined with medications, they suggest
Magnetic stimulation scores modest success as antidepressant



0 comments :
Post a Comment