This certainly is good news for patients who have have cancer in this area of the body as it will allow for easier swallowing and have a better quality of life. It can also be used to correct issues that have perhaps developed as result of surgical procedures related to removing a tumor.
We are all familiar with heart stents but they are being used with other parts of the body as well. Not too long ago I spoke with Cook about their stents being used to treat PAD in the legs too and that can be life saving before a surgical procedure.
Cook Medical Interview Discussing PAD Leg Therapies– Rob Lyles, VP Peripheral Intervention Division
In the area of hospital acquired infections, they also have a line of catheters that are preloaded with antibiotics that also help cut down bloodstream infections. BD
Catheter-Related Bloodstream Infections - Interview
WINSTON-SALEM, N.C.--(BUSINESS WIRE)--Cook Medical’s controlled-
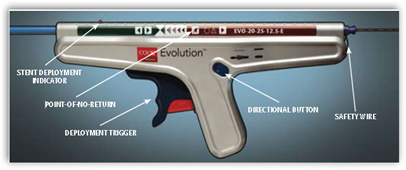
release esophageal stent fully covered in silicone has received 510(k) clearance from the U.S. Food and Drug Administration (FDA), making available yet another advanced solution from Cook Medical, the only full-line supplier of endoscopic devices. The Evolution® Controlled Release Esophageal Fully Covered Stent is designed to improve quality of life for patients with malignant esophageal strictures and tracheoesophageal fistulas (TEF) by improving the patients’ ability to swallow, and its silicone that fully covers the stent helps prevent tumor ingrowth. In addition, the system gives physicians unparalleled control during stent deployment and recapture.
“The addition of the fully covered stent to Cook's existing Evolution product line provides more options to physicians to treat their esophageal cancer patients.”
Esophageal cancer is among the most rapidly increasing malignancies in North America. More than 16,500 new cases of esophageal cancer are diagnosed annually in the U.S., most associated with risk factors such as alcohol consumption, tobacco use, GERD (gastroesophageal reflux disease) and obesity1. The vast majority of patients die within a year of diagnosis. An esophageal stent is typically used to open a passage obstructed by a tumor that has advanced beyond surgical treatment. The stent improves the patients ability to eat and swallow, allowing them to maintain a better quality of life even in the end stage of the disease. Stents are also used to treat TEF (tracheoesophageal fistula), an abnormal connection between the trachea and esophagus that can occur congenitally or develop in adults after laryngeal surgery
The Evolution Fully Covered Stent is an esophageal stent with silicone internal and external of the stent that resists tumor ingrowth and makes swallowing more comfortable for the patient. It’s also easier to place because the physician does not have to deduce where the covering ends to position it properly in the center of the tumor. The fully covered Evolution is also designed to secure the stent in the esophageal tract, potentially reducing the risk of stent migration after placement and eliminating the need for repeat procedures.



0 comments :
Post a Comment