This is a bit of complicated issue for many as to setting up guidelines and rules that can be used, simply because technology in Health IT is bursting at the seams with 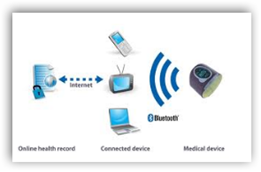 new technologies and software being created almost daily. Back in April the first rules on MMDS for Class 1 devices was announced and there’s still gray here to and with the multitude of apps and what they do today, the medical apps that will or will not be approved by the FDA is certainly leaving a lot of room for discussion.
new technologies and software being created almost daily. Back in April the first rules on MMDS for Class 1 devices was announced and there’s still gray here to and with the multitude of apps and what they do today, the medical apps that will or will not be approved by the FDA is certainly leaving a lot of room for discussion.
FDA Rule Regarding Medical Device Data Systems Takes Effect–MMDS (Medical Device Data Systems) Rule For Class 1 Devices
Dr. Halamka created his opinions and thoughts here at his blog and you can read the entire post here, but again when consuming all of this, he has some questions and issues too so we don’t have a real definitive policy yet. Some of the obvious apps that are not regulated as he mentions are e-books, etc. in other words reference  materials not directly related to a specific patient and there’s tons of those apps floating around out there, more than I care to talk about sometimes. As he mentions the Continua Alliance definitely has some input here as relates to wireless devices too.
materials not directly related to a specific patient and there’s tons of those apps floating around out there, more than I care to talk about sometimes. As he mentions the Continua Alliance definitely has some input here as relates to wireless devices too.
Global Certification Forum and Continua Health Alliance to Collaborate to Certify Personal Health Records - Wireless Devices
In addition this also puts the FDA in the same spot as private industry in having to make sure they have enough engineers to facilitate approval for apps, so again staffing and availability of personnel, one more consideration to think about, all in house, contract or a bit of both? A few decisions impact more than just one area here and then the agency has the old battle with Congress in not understanding all the agency does.
FDA and Medical Devices-Who Doesn’t Get This, They Are Looking for Engineers Just Like Technology Companies Are Doing- Get Some Congressional Digital Literacy in Place
Who knows, we could end up with an all or nothing arrangement here:) I don’t the “nothing” portion though is going to fly. Ambiguities are mentioned so in this area you have 100 different apps basically doing the same thing too, well maybe not that many but a ton of apps that only “do one thing” which drives me nuts as well as I think aggregated and collaborative efforts here need to be accelerated as a consumer I don’t want to learn more apps than I have to. The smartphone folks who make the phones are off the hook and that was a big one, so again it’s back to the software and the algorithms.
A bit of satire here, but I think they all should be FDA approved and this way we can keep the number of apps smaller for those that duplicate what 50 other apps do! Of course that would lead to several levels of approval, but nothing with that as far as I am concerned and the FDA would not be caught blindsided and would have the opportunity to look at each one and the agency might actually help out consumers with knocking out so many duplicate types of programs that are out there in mHealth, so I say let the FDA look at all of them and the bonus points are better apps with less duplication. In addition, the FDA would also be able to constitute a list too of who’ selling data here as well…and we all want to know that.  It’s almost like approving a new drug and generics except this time it’s mHealth apps:)
It’s almost like approving a new drug and generics except this time it’s mHealth apps:)
Give the FDA the engineers and software folks they need and have them look at all apps and this way we have no blind sides either and tier the levels of approval maybe. With the medical records portions of approval, they could use the ONC certification information as being justified for their approval so the 2 agencies could collaborate in unique and new fashion. Anyway, that’s my thought here.
I make this comment as many mHealth apps will be working with medical records so it’s a CYA for the FDA to ensure the applications are correctly working with certified EHRs and could offer a nice check and balance. I say this not to be a hassle for medical record vendors at all, but it may help them too with knowing what apps are being written out there to connect with their data too and be on record with it, in short, good record keeping on data. At minimum, having a listing of all the current medical record vendors at the FDA will supply data that someday will be creeping in to this arena just as mHealth technology develops and you can’t stop that.
The more I think about it, the more I like the idea of the name brand and generic thought processes with mHealth medical apps!. We can just call those that duplicate the same information “clone apps” and verity the algorithm structure as performing the same function as the original application on file as long as the same type of data is the end result and no patent interference at this level please as this is just a “working” stamp of approval for entirely different purposes.
Get the FDA the funding and engineers they need to make it happen and we will have less “crap” software floating around out there. After reading my comments please continue on to read what Dr. Halamka has to offer from a higher level:) BD
The FDA will not seek to regulate mobile medical apps that perform the functionality of an electronic health record system or personal health record system. However, the FDA defined a small subset of mobile medical apps that may impact the functionality of currently regulated medical devices that will require oversight. Here's a thoughtful analysis by Bradley Merrill Thompson of Epstein Becker Green, which he has given me permission to post:
"Today, FDA published the long-anticipated draft guidance on the regulation of mobile apps—more specifically, what the agency calls “mobile medical apps”. This draft reflects significant efforts by FDA in a fairly short amount of time, and we applaud that work. Much of the framework of the FDA guidance is consistent with the work the mHealth Regulatory Coalition (MRC) published on its website earlier this year (www.mhealthregulatorycoalition.org). While FDA has done a good job getting the ball rolling, there are a number of areas that require further work. We all (including FDA) recognize that this draft guidance is certainly not the end of the story.
This draft guidance has no doubt generated a ton of questions. So, the timing of the release is perfect! On July 27th, a large group of individuals involved in the mobile health space will be congregating at the Continua/ATA Summit to discuss regulation of mHealth products. Bakul Patel, the mobile health policy guru at FDA, will be there to discuss the details of this guidance, as will the mHealth Regulatory Coalition, which is set to release its own version of proposed mHealth guidance in the coming weeks. The discussion will surely be lively and informative. There’s still time to register and I hope to see you there."
Life as a Healthcare CIO: FDA Mobile Medical Applications NPRM



0 comments :
Post a Comment