The Heartware system is finally available in the US. It has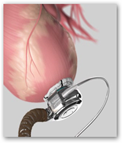 already received the European CE approval and over 2,000 people outside the US have already been implanted with the device. The device is primarily for those who are waiting for a heart transplant that have end state left ventricular heart failure. The first hospitals to get the device are those that participated in the clinical trials in the US. This was monitored by the FDA and more on that topic can be read below and the device has been available outside the US since 200
already received the European CE approval and over 2,000 people outside the US have already been implanted with the device. The device is primarily for those who are waiting for a heart transplant that have end state left ventricular heart failure. The first hospitals to get the device are those that participated in the clinical trials in the US. This was monitored by the FDA and more on that topic can be read below and the device has been available outside the US since 200
HeartWare Receives Approval From the FDA Authorizing 94 Additional Patients for Clinical Trials with the HVAD Mini Heart Pump

The power sources are external. This is really an engineering feat to say the least. The pump is the size of a golf ball. An external controller is worn like a belt or over the shoulder like purse. The video below tells you a bit more about how this works and compliance and debugging the device is needed by the patient. BD
The Food and Drug Administration on Tuesday approved a new heart pump for patients with severe heart failure who are awaiting a heart transplant.
awaiting a heart transplant.
Regulators approved HeartWare's Ventricular Assist System, a battery-powered device that is implanted in the chest, where it helps the heart's lower left chamber pump blood throughout the body.
The FDA has previously approved similar devices, known as ventricular assist devices, but HeartWare's device is smaller and may be easier to implant in some patients.



0 comments :
Post a Comment