The video explains the difference between adhesives and sealants and I learned something by watching the video as well.  Not having to go home with drainage tubes as a patient, this is huge, I don’t care who you are, if that’s an option, I think everyone would be for it.
Not having to go home with drainage tubes as a patient, this is huge, I don’t care who you are, if that’s an option, I think everyone would be for it.
TissuGlu can now begin marketing the product to hospitals. It is a two part material with part A and part B that need to be mixed together. It begins to cure with moisture.
TissuGlu already has approval in Europe. It looks like a glue gun to put you back together again. TissuGlu was developed by Eric Beckman, University of Pittsburgh chemical engineering professor and it consists of amino acids which are dissolved in the body.
“Cohera Medical’s recently completed ‘No Drain’ study confirms that TissuGlu is a clinically superior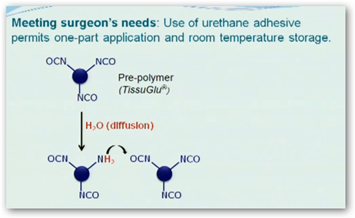 alternative to closed-suction drains in large flap procedures such as abdominoplasty. In the pivotal clinical trial, when TissuGlu was used, patients required fewer post-operative treatments and resumed normal activities, such as going to work, showering and using the stairs, more quickly.
alternative to closed-suction drains in large flap procedures such as abdominoplasty. In the pivotal clinical trial, when TissuGlu was used, patients required fewer post-operative treatments and resumed normal activities, such as going to work, showering and using the stairs, more quickly.
Currently, most patients who undergo large flap procedures have drains inserted to remove fluid that accumulates under the skin at the surgical site. Drains are often uncomfortable for the patient and can lead to additional complications. TissuGlu forms a strong bond between tissue layers, helping to reduce the space where fluid can accumulate during healing.”
Cohera Medical only has 22 employees but I would imagine they are destined to grow quickly once the product is being shipped and used by hospitals.
This is exciting stuff and no refrigeration is required either. BD
A small North Side company, newly bestowed with FDA approval for its ground-breaking internal surgical adhesive, believes it may be tapping into a billion-dollar market.
Cohera Medical Inc.’s lead product, TissuGlu, will be used to connect tissue flaps following major abdominal surgery, eliminating the need for patients to go home with drains protruding through their skin. Earlier this month, the company received the FDA go-ahead to begin marketing the product to U.S. hospitals.
President and CEO Patrick Daly sees the wide-scale availability of TissuGlu as a win all around: Patients won’t face the discomfort and infection risk associated with drains, while hospitals will save money as a result of lower complication rates and fewer re-admissions.



0 comments :
Post a Comment