Hard Hat area: "Using WinNonlin AutoPilot, customers can automate the production of PK analyses mandated by the FDA for any new drug submission, including business rules and formats"...."Pharsight software tools are used extensively by leading pharmaceutical and biotechnology industry customers, academic institutions, and important regulatory authorities such as the Food and Drug Administration (FDA)...data helpful in bringing new drugs to market....BD
MOUNTAIN VIEW, Calif., March 10 /PRNewswire-FirstCall/ -- Pharsight Corporation , a leading provider of software and strategic services for optimizing clinical drug development, today announced that Procter & Gamble has purchased floating licenses of WinNonlin AutoPilot to use in conjunction with their licenses of WinNonlin.
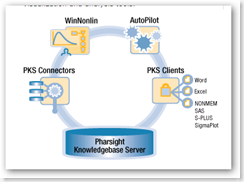
WinNonlin AutoPilot is configurable software that pre-clinical and clinical research scientists can use to automate common or repetitive tasks during clinical pharmacokinetic (PK) analysis and to create report-ready tables and graphs. Using WinNonlin AutoPilot, companies can increase their productivity while improving the quality and consistency of analyses and reports.
AutoPilot orchestrates PK analyses by selecting input data from a user's local file system or Pharsight Knowledgebase Server(TM) ("PKS"), and then directs WinNonlin to perform analyses and produce report quality tables, figures, and text output (e.g., in Microsoft(R) Excel(TM), SigmaPlot(R), and Microsoft Word(TM) for regulatory submissions and interim reports.
"We have a rapidly growing customer base of companies that use Pharsight's products and services. All of the world's largest 50 pharmaceutical companies license at least one of Pharsight's computer-assisted drug development products, and 22 of the world's top 50 pharmaceutical firms currently utilize the company's Strategic Consulting Services group in multiple therapeutic areas."



0 comments :
Post a Comment