Warfarin is the second-most-likely drug, to cause adverse events requiring hospitalization. The use of warfarin sensitivity testing in the U.S. could avoid 85,000 serious-bleeding incidents. More in the way of personalized medicine and if you were a patient determined to have an adverse reaction to the drug, this perhaps could be lifesaving to make sure a wrong dose is not delivered or perhaps if it should be used. BD
“ NEW YORK (GenomeWeb News) – The US Food and Drug Administration has cleared for arketing Osmetech’s eSensor Warfarin Sensitivity Test, the London-based firm announced today.
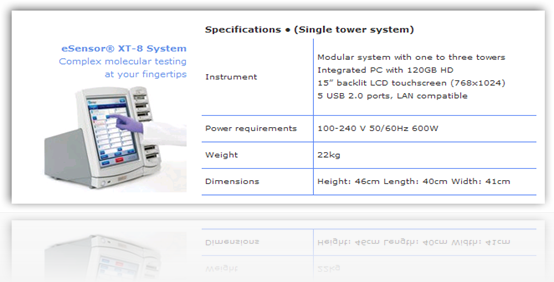 The genetic test is used to aid physicians in identifying patients who are at risk for increased sensitivity to the blood-thinning drug warfarin. Osmetech submitted its application with the FDA for the test at the beginning of this year. The firm said that the FDA also cleared its second-generation eSensor XT-8 molecular diagnostics platform.”
The genetic test is used to aid physicians in identifying patients who are at risk for increased sensitivity to the blood-thinning drug warfarin. Osmetech submitted its application with the FDA for the test at the beginning of this year. The firm said that the FDA also cleared its second-generation eSensor XT-8 molecular diagnostics platform.”



Hey, I love a new gadget as much as the next guy, but I am so unexcited by warfarin genetic testing. It won't affect my management at all--my warfarin management in the office is consistent with University of Michigan protocols. The protocol is simple: if the patient is uncontrolled, bring him back soon. If he's under control, he can come back in a few weeks. This will automatically control for the "sensitive" patients in that they are just followed more closely.
ReplyDeleteBesides not helping me care for patients, the warfarin sensitivity testing has another problem: we may be out of the warfarin business in a few years. There has been increasing testing of second-generation drugs, and several are nearing approval. I wrote about one of these on my website:
Thank for the update. I can certainly always use updated and current information, especially as relates to how drugs are use and will be used.
ReplyDeleteGood to know since the risks associated with warfarin seem so high as well.