The devices have previously been cleared for other treatments so this adds more functionality to the device. This is the first minimally invasive tool approved to actually kill the cells that contribute to causing heart failure. One thing for certain here, writing this blog is giving me quite an extensive education in areas of cardiology and endovascular surgery by all means and I can now understand the detailed procedures that are followed with the devices as well. The areas of treatment for the heart just like anywhere else in our body are getting to be very specific and appear to require a much more detailed diagnosis than let’s say what was done 10 years ago. BD
Feb. 6 (Bloomberg) -- Johnson & Johnson won U.S. approval to sell the first devices to burn away rogue cells that cause the most common form of irregular heartbeat.
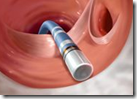
The Food and Drug Administration cleared NaviStar ThermoCool and EZ Steer ThermoCool Nav as the first minimally invasive tools to ablate, or kill, the cells that trigger atrial fibrillation, the agency said today in an e-mailed statement.
Atrial fibrillation affects more than 2 million Americans, causing debilitating fatigue for some and raising the risks of strokes, heart attacks and death, according to the U.S. National Institutes of Health. The heart-ablation market may reach $2 billion in annual sales by 2011, said Rick Wise, a Leerink Swan & Co. analyst in New York, in a note to clients last year.



0 comments :
Post a Comment