Not too long ago I had an extensive interview with the Leg Therapies Division of Cook Medical too, follow the link below for the full story.
Cook Medical Interview Discussing PAD Leg Therapies– Rob Lyles, VP Peripheral Intervention Division
“I posed Rob with a scenario, and asked whether I would have the same type of surgery on my leg as my heart if I were a PAD patient.
Rob said that actually, the arteries and veins in the legs are a bit more complex. Aside from pumping the heart is stationary, whereas the legs are constantly moving. We can still approach treating the legs in a minimally invasive way though. With placing a stent in the leg, extra care for durability is a big concern as again, the leg will be moving and the device needs to remain in place and not slip or move.”
This procedure can save legs and potentially avoid amputations, so take a look at this one. BD
Press Release:
”Workhorse” device completes suite of low-profile PTA balloons, provides physicians with enhanced deliverability to the superficial femoral, iliac and renal arteries
Bloomington, Ind., July 15, 2009 – Cook Medical has received clearance from the U.S. Food and Drug Administration (FDA) to market its newest balloon dilatation catheter, the Advance® 35LP. The device is intended for use in patients with lesions in the femoral, iliac and renal arteries and rounds out Cook’s complete line of low-profile PTA balloons.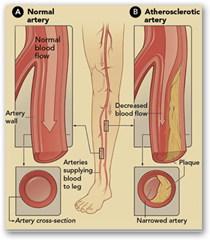
The Advance 35LP will play an integral part in Cook’s Leg Therapy Program, an initiative geared toward helping physicians address the anatomical challenges of treating Peripheral Vascular Disease (PVD) and deliver clinically effective interventional solutions to the patients who need them most. Commonly associated with diabetes, hypertension and coronary disease, PVD can result in amputation or death if left untreated. Few are aware of the minimally invasive treatment options available apart from amputation or surgical bypass, the latter of which is the current gold standard in treating this disease.
The Advance line comprises three low-profile balloons (14LP, 18LP, 35LP) that range in size and composition to treat lesions in the peripheral arteries as well as obstructive lesions of native or synthetic arteriovenous dialysis fistulas. The Advance 35LP balloon, compatible with a .035-inch wire guide, is designed to address common above-the-knee blockages. The Advance 18LP, compatible with a .018-inch wire guide, is geared toward the femoral artery and popliteal region of the leg. The Advance 14LP, compatible with a .014-inch wire guide, is reserved for treatment of the most tortuous anatomies of the lower leg, including the popliteal and infrapopliteal arteries.
Each balloon features a low crossing profile and small-sheath compatibility, which helps reduce the need for an invasive arterial entry and may shorten patient recovery time. Advanced thermal setting of the balloon folds improves rewrap and sheath pull-back, and a unique double-lumen shaft construction using optimized nylon-blend material reduces balloon inflation/deflation time and improves pushability while maintaining kink resistance.
About Cook
Cook Medical was one of the first companies to help popularize interventional medicine, pioneering many of the devices now commonly used worldwide to perform minimally invasive medical procedures. Today, the company integrates minimally invasive medical device design, biopharma, gene and cell therapy and biotech to enhance patient safety and improve clinical outcomes in the fields of aortic intervention; interventional radiology; critical care medicine; gastroenterology; peripheral vascular medicine; bone access and oncology; interventional cardiology; general surgery and soft tissue repair; urology; and assisted reproductive technology, gynecology and high-risk obstetrics. Founded in 1963 and operated as a family-held private corporation, Cook is a past winner of the prestigious Medical Device Manufacturer of the Year Award from Medical Device & Diagnostic Industry magazine. For more information, visit www.cookmedical.com.
Related Cook Medical Reading:
Cook Medical Unveils MicroWires to support Leg Therapy - Peripheral Arterial Disease



0 comments :
Post a Comment