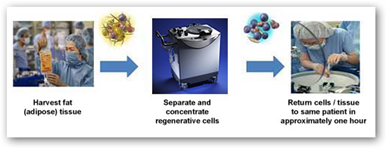
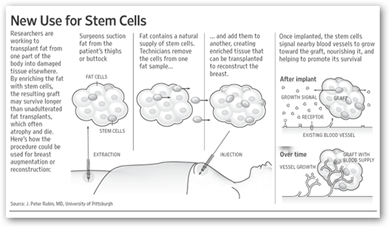
I have written a couple times about this procedures that takes your own stem cells and harvests them in a device and then returned to the patient’s  body. This process has been used in Japan for breast reconstruction for a couple years and you can read below that GE has also invested. It takes the device about an hour to clean and make the stem cells available from other fat in the body. This could impact the way breast reconstruction is done in the future. BD
body. This process has been used in Japan for breast reconstruction for a couple years and you can read below that GE has also invested. It takes the device about an hour to clean and make the stem cells available from other fat in the body. This could impact the way breast reconstruction is done in the future. BD
Cytori Therapeutics -natural alternative to breast implant technology
GE Healthcare to Commercialize Cytori Therapeutics Stem Cell Technology – Regenerative Medicine
Jul 17, 2009 (SmarTrend(R) News Watch via COMTEX) ----Cytori Therapeutics (NASDAQ:CYTX) received notification from the FDA that the Celution 700 system will be approved as a medical device under the Federal Food, Drug, and Cosmetic Act. This will allow Cytori to submit an application to market the Celution 700 to be used in aesthetic contouring and filling of soft tissue voids. According to Cytori's press release, CEO Christopher J. Calhoun, said, "This important decision provides greater clarity of our regulatory path in the U.S. and is consistent with our interpretation of current device regulations.
We are preparing for the next steps in the process of working with the FDA to determine the specific device marketing application to submit, including whether clinical evaluations will be necessary."



This headline is misleading. The FDA has not given notice that Celution 700 will be approved. It has notified that the company that if they want to get approved, they should submit a PreMarket Approval Application (PMA) for a DEVICE, not a New Drug Approval (NDA) application for a DRUG.
ReplyDelete