I have to say in his defense though, that position is one tough job as it has evolved over the last couple of years and it’s going to take someone with both clinical and IT knowledge to fill those shoes. Devices and their capabilities are growing at a phenomenal rate, and so are the types of devices.
In this position too, it is difficult to discuss details with a Congress that has less than adequate IT knowledge too and perhaps “dummy down” the 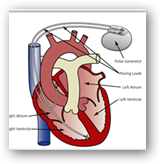 conversations. How can the person intelligently discuss new devices that report data and connect to personal health records to a group that back in January, the Senate committee on the medical stimulus project, that had never seen a PHR or taken 30 minutes to to a search on the web! Here’s an example below, I wonder if anyone in Congress knows what this device can do? Do you think any of them might try it out for size, even to collect and tally their weight using a simple example?
conversations. How can the person intelligently discuss new devices that report data and connect to personal health records to a group that back in January, the Senate committee on the medical stimulus project, that had never seen a PHR or taken 30 minutes to to a search on the web! Here’s an example below, I wonder if anyone in Congress knows what this device can do? Do you think any of them might try it out for size, even to collect and tally their weight using a simple example?
FDA approves HealthPal – Bluetooth Device that Collects from Other Reporting Devices and Sends Information to PHR – HealthVault or Google Health
Or maybe this device:
FDA Approves PDA Size Portable Ultra Sound – Signostics
And here’s one more device that sends email and it’s an implant!
Biotronik Home Monitoring – Cardio Implant that sends email, SMS or faxes
It’s a tough job and there’s now talk of the FDA approving some cell phones if you have not seen this, as they are becoming “medical devices” that are collecting data, and with blue tooth it’s getting complicated to classify exactly where they belong and that is a lot of responsibility to have on one’s plate, thus a lot of both clinical and IT knowledge is really needed today and hopefully our leaders can play catch up to expedite matters and bring this full circle, participation and leaving the 70s would help a lot. BD
FDA may regulate certain mobile phones and accessories – Somebody Finally Agrees with Me
Daniel Schultz, the director of the device division at the Food and Drug Administration, said Tuesday he is resigning "by mutual agreement" with FDA leaders.
In a letter to his section's employees, Dr. Schultz said he made the decision "based on discussions with Commissioner [Margaret] Hamburg." He has worked at the FDA's Center for Devices and Radiological Health for 15 years and led it for the past five years.
Dr. Schultz's decision-making in recent years has faced criticism in Congress and by some FDA scientists and doctors. Sen. Chuck Grassley (R., Iowa) held hearings two years ago on Dr. Schultz's approval of a brain-stimulation device over the objections of several FDA doctors. Mr. Grassley complained at the time that science was being ignored in favor of industry. Dr. Schultz said his decision had been based on sound medical data.
An FDA spokesman said Dr. Schultz's decision came as the result of talks with Dr. Hamburg and had nothing to do with any specific issue related to a device's approval process.
FDA's Device Chief Will Step Down - WSJ.com



0 comments :
Post a Comment