I said this a while back, but most individuals may not be up to date on what and where the classifications are for medical devices. Sure we all may know about a knee, but how about those that report data? Well soon that knee may be doing that too. It’s not that far away folks so wake up smell the roses.
Life Sustaining Medical Devices – How Dependable are They Says the FDA
If you hang around this blog often enough, you have read about the blue tooth inhaler for one, that seems to be my favorite reference as it makes the point quickly. You don’t inhale or forget, the device text messages you, and then it emails you, so they nag as well as record data. Where will this be down the road, will insurance companies have access to seeing if you inhale the 3 designated times during the day and not pay a claim based on missing a few times? Think about it, and this is why you want a PHR so you have control, otherwise who knows where it will go. Devices are smart and report information. They have been out there for a while now and are getting smarter every day. 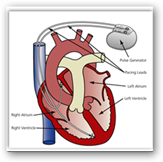
There are implanted defibrillators that do the same, like Merlin from St. Jude. Here’s one more….Biotronik Home Monitoring – Cardio Implant that sends email, SMS or faxes
- Use Merlin.net PCN to centralize device data from remote follow-ups, in-clinic and post-implant programmer sessions
- Export all patient device data from Merlin.net PCN directly into your EHR
- Connect to the first cardiac rhythm management company to pass EHR standard guidelines set forth by IHE (Integrating the Healthcare Enterprise)
- Access your patients' device information anytime, anywhere
- Enable patients to transmit from the comfort of their homes or while they are traveling
- Allow transmitters to be placed in remote clinics, nursing homes, and emergency rooms
- Reduce the number of office visits, providing greater convenience and flexibility for your patients
St.Jude Collaborates With Microsoft HealthVault
The next move for the FDA too might just be in the EMR/PHR area too, think about it. BD
So at a high-level, we look for two things: (1) a device with (2) a medical intended use. The first prong of the test — that there must be an actual product — means FDA doesn’t regulate, for example, medical procedures. The thing in question must be a thing, and not information or something else intangible. Software can be a medical device if it’s written on computer media, as opposed to printed on paper. The media with the code written on it is enough of a “thing” for FDA to regulate.
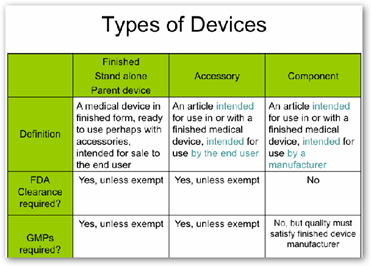
That makes a big difference in terms of applicable regulatory requirements. Components are exempt from most FDA regulatory requirements, with the regulatory burdens being borne by the finished device manufacturer. Accessories, on the other hand, since they go right to the end user, must meet the FDA requirements before they leave the hands of the accessory manufacturer. These differences are summarized in Table A below:
If it is a medical device, what next?
This analysis only answers the threshold question of whether an article is a medical device. If it turns out to be a regulated article, a second step is to figure out the degree of that regulation. A fair number of medical devices are exempt from FDA premarket clearance, and others are exempt from the obligation to employ good manufacturing practices. The risks associated with the intended use determine the level of regulatory requirements, including validation and other design rigor that FDA would require.
FDA may regulate certain mobile phones, accessories | mobihealthnews



0 comments :
Post a Comment