Cook Medical is presenting some very interesting and favorable numbers in reference to their clinical trials. If you check in here often enough you will find all kinds 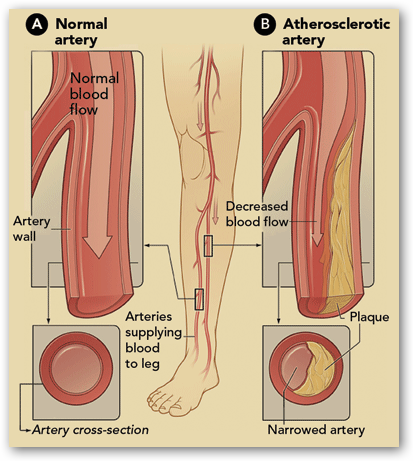 of information about Cook Medical and frankly I have learned more about stents, PAD, Leg Therapy and more than I ever thought I would need to know. Mentioned here is Rob Lyles and he and I had a chance to talk last year about PAD Leg therapies. This is good stuff to know about and in the interview he goes into detail as to how the Zilver stent is used in legs now to avert blood clots. The leg stents have to be a bit more rugged in design, as your legs are moving all the time. The stent is coated with paclitaxel, a cancer drug. The drug lasts in the body for 6-8 weeks, and is used to help prevent infections as well as decrease the chance of the body rejecting the stent. You can read more here:
of information about Cook Medical and frankly I have learned more about stents, PAD, Leg Therapy and more than I ever thought I would need to know. Mentioned here is Rob Lyles and he and I had a chance to talk last year about PAD Leg therapies. This is good stuff to know about and in the interview he goes into detail as to how the Zilver stent is used in legs now to avert blood clots. The leg stents have to be a bit more rugged in design, as your legs are moving all the time. The stent is coated with paclitaxel, a cancer drug. The drug lasts in the body for 6-8 weeks, and is used to help prevent infections as well as decrease the chance of the body rejecting the stent. You can read more here:
Cook Medical Interview Discussing PAD Leg Therapies– Rob Lyles, VP Peripheral Intervention Division
Metal Stent Market – Study Compares Plastic to Metal
Cook Medical Unveils MicroWires to support Leg Therapy - Peripheral Arterial Disease
Some of this medical technology can save patients from amputations and increase their lives. Patients who have had limbs amputated have shown to have a shorter life when PAD is in the picture. BD

Press Release:
Trial data, panel sessions, demonstrations and simulations highlight clinically proven treatment and diagnostic solutions for entire peripheral vasculature
Miami, Fla. – January 19, 2009 – Signaling its continued commitment to addressing the anatomy of the entire peripheral vasculature and combating disease with targeted diagnostic and interventional devices, Cook Medical is presenting its latest advancements at the ISET 2010 International Symposium on Endovascular Therapy. In addition to live and simulated device demonstrations in booth #208, Cook’s activities at the meeting include data presentations from two ongoing clinical trials and breakout sessions on common and debilitating conditions such as varicocele.
“The number of people affected by some form of peripheral vascular disease is increasing at alarming rates worldwide,” said Rob Lyles, vice president and global leader of Cook’s peripheral intervention business unit. “Awareness is a fundamental line of attack for us against these spiking numbers. It is our mission to inform patients and physicians alike about the intricacies surrounding treatments that span the entire vascular disease spectrum and arm them with the technologies needed to improve outcomes.”
Findings from the REFORM clinical trial evaluating the Formula™ Balloon Expandable Stent for the long-term treatment of renal artery stenosis following suboptimal angioplasty are being presented by Dr. Robert Bersin, medical director of endovascular services at Seattle Cardiology and Swedish Medical Center. Early data from the single-arm, 100-patient study suggest high technical and procedural success rates, clinically significant reductions in systolic blood pressure at one month, stable renal function, and no evidence of safety concern. Clinical follow-up will continue through three years, and enrollment for the European arm of a trial evaluating a drug-eluting version of the Formula stent for renal indications is scheduled to be announced at the LINC meeting in Germany later this month.
“Cook has a long legacy of improving patient outcomes by driving the development of innovative new treatments backed by sound, clinical evidence,” said Mark Breedlove, director, peripheral intervention strategic technology, Cook Medical. “Last year, we changed the landscape for peripheral arterial disease with the launch of our leg therapy program and the introduction of our Zilver® PTX® drug-eluting stent platform. This year, we’ll advance our support of physicians treating other vascular conditions, such as renal disease and atherosclerotic lesions in areas of the body beyond the legs.”
Additional data are being presented at the show from the registry arm of Cook’s ongoing Zilver PTX trial. The data show that 82 percent of patients treated with Cook’s Zilver PTX stent were free from reintervention at two-year follow up. The findings, presented by global principal investigator Dr. Michael Dake, medical director of the Cath/Angio Laboratory at Stanford University Medical Center, also showed significant improvement through 24 months in clinical measures including ankle-brachial index, Rutherford score, walking distance and speed scores.
Varicocele, another vascular disease targeted by Cook, was featured in Monday’s “Resurgence of Varicocele Embolization: Putting the Patient First” symposium. Dr. Lindsay Machan from the University of British Columbia and Dr. Robert I. White from Yale University and the Yale Vascular Malformation Center discussed recent advances in embolization as a line of treatment against this debilitating disease, which affects between 15 and 20 percent of men in the U.S.[1]
For more information on clinical trial results and Cook’s line of products such as the Advance® PTA line of balloon dilatation catheters, the NavAlign™ IVC Filter Delivery System and Nester Embolization Coils, visit booth #208 at ISET 2010.
About ISET:
Now in its 22nd year, the leader in interventional education continues to meet the needs of physicians who recognize the challenges that lie ahead and actively seek the knowledge to meet those challenges. ISET provides the tools – in the form of unbiased, professional education – to lead practitioners into the future of an ever-evolving field. For more information, visit www.iset.org.
About Cook Medical
Founded in 1963, Cook Medical helped invent and popularize interventional medicine, pioneering many of the devices now commonly used worldwide to perform minimally invasive medical procedures throughout the body. Today, the company integrates minimally invasive medical devices, drugs and biologics to enhance patient safety and improve clinical outcomes. Since its inception, Cook has operated as a family-held private corporation focused on providing superior patient outcomes while reducing health care costs. For more information, visit www.cookmedical.com. Follow Cook Medical on Twitter at twitter.com/cookmedicalpr.
[1] http://www.pennmedicine.org/int_rad/health_info/embol.html#what
Related Cook Medical Reading:
Cook Medical Unveils MicroWires to support Leg Therapy - Peripheral Arterial Disease



0 comments :
Post a Comment