The world of interventional procedures certainly keep growing. The procedures are much easier for the patient to withstand versus full open heart surgery, but with  most interventional pulmonary and or stent procedures, there is a trade off, you need to continue repeat visits to the doctor to ensure the device is still working properly, has not moved, etc. With full open heart surgery you are basically done, but some individuals depending on their health condition may not be able to with stand that type of surgery, so interventional procedures are the answer. A competitor with Medtronic in the pulmonary area, Edwards Life Sciences received FDA approval for aortic valve replacements with the same type of interventional procedure.
most interventional pulmonary and or stent procedures, there is a trade off, you need to continue repeat visits to the doctor to ensure the device is still working properly, has not moved, etc. With full open heart surgery you are basically done, but some individuals depending on their health condition may not be able to with stand that type of surgery, so interventional procedures are the answer. A competitor with Medtronic in the pulmonary area, Edwards Life Sciences received FDA approval for aortic valve replacements with the same type of interventional procedure.
Edwards Lifesciences Receives FDA Approval For Magna Ease Aortic Valve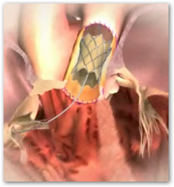
Last year Medtronic also purchased 2 other heart valve companies which market products outside the US.
Medtronic to Buy 2 Heart-Valve Companies
When you look at how this device operates, you can see a tremendous amount of detail here, first with the valve and then the catheter itself working. All through out any material I have seen today on the Melody valve, there seems to be a warning on how the valve could in rare cases break, like we hear about drugs so medical devices now have small potential side effects that can have the same results relating to death as well.
We are moving along so well with all of this pulmonary technology and sometimes I wonder if an aortic valve and a pulmonary valve could live together in the same heart, but no doubt this has been tried with rats, etc. you could almost bet if not ready for prime time medicine yet.
The Aortic Valve replacements are still the procedure in most demand and the number of pulmonary valves is just a small shadow by comparison but both eliminate the need for open heart surgery through interventional procedures. Come to think of it, even Dr. Oz did a demonstration on his show on cardiac catheters. BD
The Food and Drug Administration Monday approved a Medtronic Inc. heart valve that can be implanted without open-heart surgery.
Known as the Melody transcatheter pulmonary valve, the device is designed to be implanted through a small catheter inserted into the patient's body. It replaces the pulmonary valve in patients born with a heart defect. It is the first heart valve approved for sale in the U.S. that can be implanted without open-heart surgery.
The device "allows patients to undergo a much less-invasive procedure to treat their heart condition," said Jeffrey Shuren, director of the FDA's devices division. The valve doesn't cure the heart condition, however, and over time it will likely need to be replaced.
Last year, Medtronic acquired a company that sells an aortic- valve-replacement system in Europe and plans to eventually seek FDA approval of the product in the U.S. Edwards Lifesciences Corp. also sells transcatheter heart valves in Europe and plans to seek FDA approval to sell its valves in the U.S. The company hopes to win approval for the valves next year.
The FDA said Medtronic will be required to conduct two post-approval studies to assess long-term risks and benefits of the product. The company will also be required to assess a physician-training program and to maintain a database of Melody recipients.
Medtronic's Melody transcatheter pulmonary valve wins FDA approval - WSJ.com



0 comments :
Post a Comment