Stent technology continues to grow and become much more specific especially as relates to placement and technology available for higher levels of accuracy. The FDA has just approved another device and technique to be used with repairing abdominal aortic aneurisms.
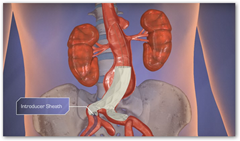
Using the Endologix technique, the procedure is a minimally invasive procedure for the patient. BD
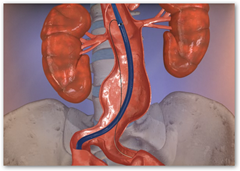
Endologix, a developer of minimally invasive treatments for aortic
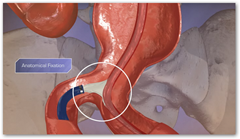
disorders, has received FDA approval for its new PowerFit Aortic Extensions. The PowerFit is designed to provide physicians with enhanced visibility under fluoroscopy to facilitate precise device placement during completion of the Anatomical Fixation endovascular repair of abdominal aortic aneurysm (AAA).
In addition, PowerFit's stent design and 24 circumferential contact points were shown in anatomical simulation studies to aid in proximal conformability and sealing.
PowerFit is designed for use with Endologix Powerlink main body bifurcated stent grafts and the IntuiTrak Endovascular System. PowerFit is expected to be available in a range of sizes indicated to treat aortic necks ranging from 18 to 32mm in diameter.
FDA Clears Endologix PowerFit Aortic Extensions - Medical Devices Business Review



0 comments :
Post a Comment