It was just last week I spent some time with Dr. Lawrence from the UCLA Gonda  Vascular Center and the link below will tell you about what they have going on there with treating PAD, lots of new technologies with non-invasive diagnosing too.
Vascular Center and the link below will tell you about what they have going on there with treating PAD, lots of new technologies with non-invasive diagnosing too.
The UCLA Gonda Vascular Center Treats PAD (Peripheral Arterial Disease)-Interview with Dr. Peter Lawrence Chief of Vascular Surgery
The FDA has given the OK to begin clinical trials on using stem cells for bone marrow injections. PAD (peripheral arterial disease) is kind of a silent disease that we may not be aware of until we get checked out. PAD is not new but the therapies to diagnose and treat certainly are. Back in June of 2009 I also spoke with Cook 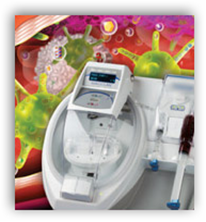 Medical about PAD and actually was my first real exposure to a full education on what it is and you can use the link below to read up there as well. Be sure and catch the part about the man walking around with a golf ball in his shoe for gosh knows how long and being the patient has lost feeling, he no idea it was in his shoe!
Medical about PAD and actually was my first real exposure to a full education on what it is and you can use the link below to read up there as well. Be sure and catch the part about the man walking around with a golf ball in his shoe for gosh knows how long and being the patient has lost feeling, he no idea it was in his shoe!
Cook Medical Interview Discussing PAD Leg Therapies– Rob Lyles, VP Peripheral Intervention Division
Ohio State University has been chosen as the first location for patient enrollment under the Assistant Professor of Surgery in the Vascular Diseases and Surgical Center. CLI (Critical Limb Ischemia) is a blockage in the arteries that restricts blood flow to the extremities and around 150,000 people a year lose a limb. Arteriocyte Medical Systems works with partner and owner Medtronic with blood component therapies.
In short this is a device that can be used at the patient bedside and offers treatment for those who are not eligible for surgery and we are talking patients with diabetes here as well with wounds that will not heal. The article states it takes 15 minutes for the process to run. The website has a patient story here for additional information on how it worked for him as he has diabetes 2. Going back to my interview at UCLA we also touched on wound care too as it all comes under the same circulation areas. Below are a couple images of before and after.
This is great news and looks to be limb saving if not life saving when you see the wounds healing and circulation coming back. Hopefully more sites will get underway soon with the trials. BD
CLEVELAND, Jan. 28, 2011 /PRNewswire-USNewswire/ -- Arteriocyte®, a leading clinical stage biotechnology company with offices in Cleveland, Ohio and Hopkinton, Massachusetts that develops proprietary stem cell and tissue engineering based therapies,
announced today approval from the Food and Drug Administration (FDA) to initiate a Phase I clinical trial using its Magellan MAR01™ technology in the treatment of Critical Limb Ischemia (CLI). The FDA Investigational Device Exemption (IDE 14522) allows Arteriocyte and its clinical partners to initiate evaluation of concentrated marrow injections (using the Magellan MAR01™ technology) in improving perfusion in ischemic tissue in affected limbs of patients with CLI who are not eligible for revascularization surgery.
The Magellan technology combines a rapid bedside tissue concentration device and sterile surgical disposables that produce platelet rich plasma from blood and bone marrow aspirations in approximately 15 minutes and was FDA approved through the 510(k) process for use as deemed appropriate by surgeons. The Magellan MAR01™ technology enables the rapid "closed system" concentration of aspirated bone marrow, yielding an injectable tissue rich in platelets, hematopoietic stem cells and mesenchymal stem cells, commonly viewed as key components in tissue repair. The company is developing MAR01™ for use as a clinical treatment for Critical Limb Ischemia, and plans to initiate additional clinical trials evaluating MAR01™ in cardiovascular disease, and the clinical setting of orthopedics and tissue repair during 2011.




0 comments :
Post a Comment