The product is under clinical trials here in the US but is available outside the US. I seem to remember having some of this done when I had my dental implants to create bone mass in my jaw, maybe not the same product but it sounds like the same or similar type of process. I know it worked. BD
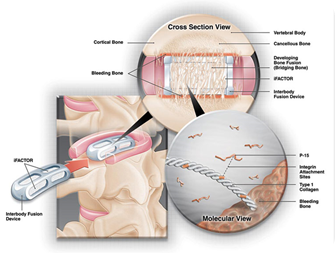
i-FACTOR™ bone graft is the only bone graft substitute that uses a unique anorganic bone mineral (ABM) and small peptide, P-15™, that
acts as an attachment factor for specific integrins in osteogenic cells. This novel mechanism of action enhances the natural bone healing process in orthopedic applications resulting in safe, predictable bone formation at a fraction of the cost of conventional growth factors.
ABM/P-15 was originally developed for use as a bone substitute in dental applications and later for orthopedic applications
The Series B financing will be used to accelerate the company’s investment in sales and marketing to meet the demand for i-FACTOR products outside the United States as well as continue to fund ongoing FDA clinical trials in the USA for spinal applications and other indications of i-FACTOR technologies under development.
http://www.cerapedics.com/intl/Home



0 comments :
Post a Comment