This is somewhat scary as with a medical device it is not a visitor, it comes to live with you. Tissue over time grows over the cables which in some instances can make the removal process even more complicated. Many have had the cables removed/replaced, but it is not a simple process. It is 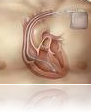 almost easier to replace a device and reconnect to existing cables, but not an option here.
almost easier to replace a device and reconnect to existing cables, but not an option here.
The built in batteries of the devices have a lifetime of around 5 years, so at that point, when replacing a device, the decision is there to replace the cables at the same time. The physicians performing the removal process state that the learning curve is pretty large too, which brings up the next issue, finding a facility and physician who is qualified and knows how to do the removal procedure of the wires.
One patient had tissue so overgrown that the wire ended up in a vein. The Sprint Fidelis was formally recalled by Medtronic, as it sometimes failed to give the life saving jolt and on other occasions the opposite occurred with a number of jolts when not needed. There are over 1000 legal cases filed and so far Medtronic has been shielded, and either Medicare or private insurance pays for the $15-20k procedure. Normally I don’t have much in the way to say about insurance coverage, but in this particular case to me it seems like it should involve some monetary compensation from Medtronic to replace the wires, and in return the patient would not go to court, it comes right back around to somebody paying the bill. BD
BOSTON — Pulling a medical device off the market is one thing. Removing it from the bodies of thousands of patients is a lot more complicated and dangerous.
Consider the Sprint Fidelis, a heart defibrillator cable. In 2007 its maker, Medtronic, stopped selling it after five patients who had the
cables died.
But only now is the full scope of the public health problem becoming clear for the Sprint Fidelis, which is still used by 150,000 people in this country.
Medtronic estimates that the cable has failed in a little more than 5 percent of patients after 45 months of being implanted. But as a preventive measure, some patients with working cables are having them removed.
Already, four patients have died during extractions. Experts fear that the toll could quickly rise if such procedures are not performed by skilled doctors at medical centers that have performed many of the operations.
Even experienced cardiologists at well-regarded hospitals, like Dr. Laurence M. Epstein at Brigham and Women’s Hospital here, consider the procedure challenging.
Dr. Epstein recently operated on a patient, a 63-year-old man, whose Sprint Fidelis cable had become so overgrown with tissue that it was stuck inside a major vein. Medtronic is supplying replacement cables, but the cost of the operation to implant a cable, which can run $15,000 to $20,000 is being borne by Medicare or private insurers.
Removing Medtronic Heart Cables Is Hard Choice - NYTimes.com
Related Reading:



0 comments :
Post a Comment