One official stated that this device stands to replace the intra-aortic balloon pump, which is an older technology. Studies are in process. The patient receiving the device was basically saved via the procedure as he more than likely could not have sustains full open heart surgery due to the weakened condition of the heart. This is an FDA approved device. ![]()
This is a fairly new procedure for the US and has been used for years in Europe. I had covered the device back in August of 2008 and since that time FDA approval has been given and there are several stories in the news about the device and here’s more detail with a patient in Texas and the newsman covering the story with one in his hand showing the power of the pump submerged in water. BD
After a day of fishing at a nearby lake in February, John Sallee arrived at his Mattoon home so out of breath it scared him.
“I could not hardly get any air in,” said Sallee, 61, a self-employed carpenter. “I was really feeling bad.”.
He experienced a health-care cost crisis of his own a few years ago when he and his wife, Karen, lost their home and most of their savings to pay for her breast-cancer treatment before she worked for Consolidated Communications and they were both uninsured.
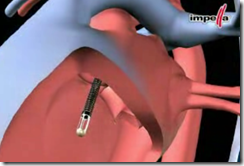
The Impella, approved by the Food and Drug Administration in 2008, is a tiny pump that is narrower than the width of a pencil and 4 to 6 inches in length. The plastic and metal pump, inserted through a catheter that is fed into an artery in the groin and snaked into the heart through the aorta, is placed inside the heart chambers by a doctor.
- Established Medicare Reimbursement codes
- Procedure code 37.68: Insertion of a percutaneous heart assist device
- Proactive reimbursement technical support including sample letters, codes, contacts, and case studies
http://www.sj-r.com/health/x1579125557/Hearts-in-crisis-boosted-from-inside
Related Reading:




0 comments :
Post a Comment