The site states there’s a treatment to use the technique and treatment to even bring your voice back to a more youthful sound, so it’s not only skin deep it appears and there’s a treatment for hands too. BD
Press Release:
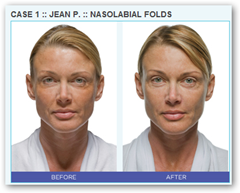
BioForm Medical Receives FDA Approval for Mixing Lidocaine With RADIESSE(r) Dermal Filler
SAN MATEO, Calif., July 16, 2009 (GLOBE NEWSWIRE) -- BioForm Medical, Inc. (Nasdaq:BFRM) today announced U.S. Food and Drug
Administration (FDA) approval for a method of mixing RADIESSE(r) dermal filler with lidocaine, which, in clinical trials, has demonstrated an improvement in patient comfort and an increase in patient satisfaction with RADIESSE dermal filler procedures. BioForm Medical will immediately begin commercial efforts to educate physicians to use this method of mixing RADIESSE dermal filler with lidocaine.
"Dermal filler procedures can create some level of patient discomfort due to injection pain. Being able to mix RADIESSE dermal filler with lidocaine has been proven to provide a significant advantage in improving patient comfort," stated Rhoda S. Narins, M.D., former President of the American Society for Dermatologic Surgery (ASDS) and a Clinical Professor of Dermatology at NYU Medical Center. "I have found in my practice that mixing with lidocaine makes RADIESSE dermal filler easier to use in many treatment areas."
This technique for mixing RADIESSE with lidocaine was developed by Mariano Busso, M.D. and the methodology was first published in the Journal of Dermatologic Surgery in June 2008. BioForm Medical conducted a 50-patient controlled clinical trial to evaluate the safety and effectiveness of mixing RADIESSE dermal filler with lidocaine. Patients in the split-face trial at two clinical sites reported that RADIESSE dermal filler mixed with 2% lidocaine was less painful than RADIESSE dermal filler not mixed with lidocaine, while providing comparable aesthetic correction. The results from the clinical trial demonstrated:
* Approximately 60% reduction in scores immediately following
treatment, measured on a Visual Analog Scale for Pain (VAS)
(p<0.0001).
* 100% of patients found RADIESSE dermal filler mixed with lidocaine
to be less painful than non-mixed RADIESSE dermal filler.
* 96% of patients found that the difference was significant enough
to affect their preference for one treatment over the other.
* Both RADIESSE dermal filler mixed with lidocaine and non-mixed
RADIESSE dermal filler were safe, with comparable local adverse
events, typical of dermal fillers. There were no serious adverse
events in the clinical trial.
* RADIESSE dermal filler mixed with lidocaine produced a comparable
aesthetic improvement as compared with treatment with RADIESSE
dermal filler without premixing.
The clinical study which supported this approval by FDA was conducted by Ellen S. Marmur, M.D., Chief of Dermatologic and Cosmetic Surgery, Mount Sinai Medical Center in New York, N.Y., and Lawrence J. Green, M.D., Assistant Clinical Professor of Dermatology, George Washington University School of Medicine in Washington, D.C.
The method approved by FDA is based on using a mixing syringe and the female-to-female luer lock connector to mix RADIESSE dermal filler and lidocaine. Components for this mixing technique are commercially available through various sources, however, in the near future, BioForm Medical will be providing them in an separate Accessory Kit as a further convenience to its customers.
About BioForm Medical, Inc.:
BioForm Medical, Inc. is a medical aesthetics company headquartered in San Mateo, California, developing products that enhance aesthetic procedures performed in dermatology and plastic surgery practices. BioForm Medical's lead product is RADIESSE(r) dermal filler, a long-lasting filler for use in facial aesthetics. BioForm Medical is developing several future aesthetics products, including a radiofrequency treatment to reduce nerve function in the forehead, a sclerotherapy treatment for spider veins, and a surgical adhesive for brow lifts. For more information about BioForm Medical, please visit www.bioform.com.
CONTACT: BioForm Medical, Inc.
Can Gumus, Senior Manager, Corporate Development
650.286.4003
BioForm Medical Receives FDA Approval for Mixing Lidocaine With RADIESSE(r) Dermal Filler



0 comments :
Post a Comment