This is a very lengthy section that goes over recalls put out by the FDA. It entails many procedures and outlines what is needed to be done. The part of this that I  am concerned with is the “red tape” procedures and lack of automation and the fact that sometimes with all of this, products get missed and patients are implanted with devices that have been recalled. Why is this important? Some of those patient die needlessly! You can take your choice here, spend perhaps hours going all over the web for information or have the ability to “scan that stent” that takes less than a minute and one could be presented a screen that says “do not use –recalled” or could include new safety information just released.
am concerned with is the “red tape” procedures and lack of automation and the fact that sometimes with all of this, products get missed and patients are implanted with devices that have been recalled. Why is this important? Some of those patient die needlessly! You can take your choice here, spend perhaps hours going all over the web for information or have the ability to “scan that stent” that takes less than a minute and one could be presented a screen that says “do not use –recalled” or could include new safety information just released.
Microsoft Tags on CBS Early Show – Wake Up FDA, Pharma and Medical Device Companies –Scan Those Drugs, Medical Devices and Synchronize with an FDA Tag Data Base – Recalls, Theft Tracking and More….
This is part of my little ongoing campaign on the blog here to hopefully open some ears to some technology that is here today and can help facilitate this and be effective and simple not only for hospitals and doctors, but also for the consumer. I’m not going to repeat the dozen or so posts I have made, but rather will include what I have sent to the FDA, some device and drug companies and you can make up your mind and see what you think.
Use Tags and scan that stent before it is used in surgery –get “real time” recall information! Also a synchronized data base to ensure compliance with notifications would benefit the FDA too and take away some of the administrative nightmares with data coordination on their end. I am convinced this is a winner for everyone. I have had some nice favorable comments from pharmacists too on how it would even help track illegal copy cats and benefit the DEA with tracking stolen drugs, so why not, if we cant take time out from our enthusiasm with IPads for a few moments maybe something like this can share the lime light.
Below are a couple screenshots on what needs to be done and answers questions about recalls, and there’s quite a bit of text here to drill through.
From the FDA Website:
“A Firm’s Recall Communication
- A recalling firm has a responsibility to its consignees, anyone who received, purchased, or used the product being recalled, to:
- Promptly notify its direct accounts via a recall communication
- e.g. By issuing press releases or providing detailed instructions
- Promptly notify its direct accounts via a recall communication
- Should supply information to help users identify the product and take steps to minimize health consequences
- Identify product subject to recall
- Explain reason for the recall and hazard involved
- Further distribution or use should cease immediately
- Direct accounts should notify its customers who received the product, where appropriate
- Instructions should be included regarding what to do with the product
- The recall communication should not contain irrelevant qualifications, promotional materials, or any other statement that may detract from the message.
- Consignees that receive a recall communication should immediately carry out the instructions set forth by the recalling firm and, where necessary, extend the recall to its consignees.
- To take action to prevent the problem from happening again
- Telephone calls or other personal contacts should be confirmed by written communication and or documented in an appropriate manner.”
The Technology Answer
I get a little excited and maybe enthusiastic here, but if that’s the worst that I do I figure folks can live with that if I am trying to do something positive and save lives. The fact that the technology is free really gets me going and the fact that it works on I phones, Blackberries and Windows Mobile phones.
Tags for Use in Healthcare – Medical Stents, Medications - One Scan Away From Safety Information in Real Time
Windows Tags – Bar Coding Information Made Easy from your Cell Phone
Why are we afraid of “free” life saving technology? This same tag could be placed on drug containers too, scan it and new safety information is available as well as identifying the product is authentic with an FDA supplied key to manufacturers, encrypted. It’s wild I have written on this topic several times, had a couple somewhat angry frustrated answers from others who didn’t take the time to understand how this free technology saves lives,
Tracking Medical Device Recalls – Sounds Like A Good Place for a Microsoft Tag Data Base at the FDA
Watch the video below and pretend this is a knee or hip replacement, a stent or any other implanted device and it will make perfect sense.
They can be encrypted and this information could be stored in a “Private Cloud” too.
Counterfeit Alli Warning from the FDA – We Should Be Using Technology for Easier Identification

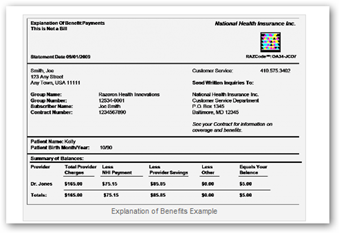
RazCode/Windows Tags – Bar Coding to Add Information to PHRs, EHRs, and More…
Of course device manufacturer’s would need to buy in too and I have emailed a few of them with my little suggestion, but it’s technology outside the normal realms maybe of what is normally received so it might meet with some passing on even looking at the potential, that I understand too. CBS did a nice story on their use as well. We can use this for marketing for sure, but why not save lives with it too and multi task a bit here it can really be beneficial.
Introduction to Medical Device Recalls: Industry Responsibilities
Here’s my Tags that are on the blog. The information also explains how the QR codes and Microsoft Tags work above. I had a doctor comment favorably too that uses them to help with his golf swing from Golf Digest Magazine.

The clip above was shown today on the CBS Early Show. CNET's Natali Del Conte discussed how QR codes may change the future of advertising and how consumers can scan the codes with their cell phones to receive information on their mobile phone. Although the segment focused mostly on the QR code, Natali did show off several Microsoft Tag examples in magazines such as Self, Details and Golf Digest, video games like Halo Wars and even a We The Kings promotional music poster. Having the Tag reader on your phone allows you to read any Microsoft Tag that you may
come across. It's easy to distinguish a Tag from a QR code:

QR Code Microsoft Tag
Wheaties uses them and the FDA might want to read up on this as other food companies may be using the codes to advertise their nutritional value, again something the FDA is responsible for monitoring too:) By the way this one in this somewhat poor image works too, I did with my phone! General Mills has been using them and I have posted too about that last year.
You can be the judge here, spend a lot of time searching for recall information on the web or be able to scan and find out in less than a minute? I know what I would prefer to do!
With setting up servers, coordinating with manufacturers, it would take a little time, but actually it could be up and running pretty fast. A Tag with an encrypted FDA emblem would be necessary to verify validity as there will be those who try to copy cat of course and this encryption process could keep those from accessing the Official FDA data base pages too, so some anti fraud mechanisms already there to be developed and put in place.
It could allow for consumers to sign up with a portal as well to be notified when a “Tag” is updated, just one more area to go here and with using some algorithms to aggregate and update information this could be pretty smooth process. Anyway, i just get excited when I see something that has the potential to cut down medical errors and save lives, which I hope there are still some like me that feel this way too.
RazCode is one company who has done some work on the encrypted gateway and adds even more to the process as the cell phone is leading up to be a way to enter data to a personal health record too. Just think patients could store a copy of their implanted device code right in their personal records to check up whenever they want. This way they know have inside their body too!
RazCode/Windows Tags – Bar Coding to Add Information to PHRs, EHRs, and More…
Wouldn’t you bet that Eli Lilly and the DEA might have enjoyed having this capability to help find the $75 million dollars worth of drugs that were stolen too? I did write to Eli Lilly with this suggestion and have not heard back yet other than an acknowledgement of receipt. BD






0 comments :
Post a Comment