These are injection kits and product is not FDA approved. Keystone had been given plenty of warnings and did not stop selling. 
It is just too bad that we don’t have bar codes here to help companies, hospitals and individuals to identify products as such. I don’t know the FDA will jump on this band wagon; however if they had entertained using 3D bar codes like Microsoft Tags for easy identification the job would be much easier and gee, do you think the FDA might enjoy an easy technology solution for compliance or is it still more effective to do this stuff the hard way? See the link below about using bar coding and Microsoft tags to enable consumers, clinicians and all to scan with a cell phone. Recalls and items like this are going to grow so the sooner the better. Take a minute to vote too if you would. BD
Microsoft Tags – Microsoft MSDN Posts Ideas from the Medical Quack About Use in Healthcare!
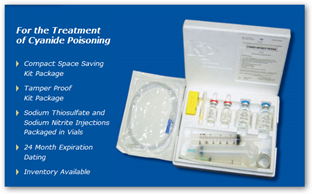
SILVER SPRING, Md., July 22 /PRNewswire-USNewswire/ -- At the request of the U.S. Food and Drug Administration, U.S. Marshals today seized $39,000 worth of products labeled as cyanide antidote kits from Keystone Pharmaceuticals in Laguna Hills, California. The seizure warrant was issued by the U.S. District Court for the Central District of California.
The cyanide antidote kits distributed by Keystone are unapproved new drugs under the Federal Food, Drug and Cosmetic Act and are therefore not permitted to be introduced into interstate commerce. The products have not been proven safe and effective for their intended use.
The kits also are misbranded because their labeling does not contain adequate directions for their use. Additionally, the seized products are adulterated because they were manufactured under conditions not in compliance with current Good Manufacturing Practice (cGMP) to assure that they meet the identity, quality, and purity standards they claim to possess.



0 comments :
Post a Comment