You would have to living under a rock today not to notice the fire that keep popping  up for the FDA as the world we live in is changing and coupled with years of neglect in the IT department, they have a lot of work and need a lot of help with other agencies as it’s not all self contained to one agency any more. Below is how they are working with the FCC and there are working agreements with the NIH.
up for the FDA as the world we live in is changing and coupled with years of neglect in the IT department, they have a lot of work and need a lot of help with other agencies as it’s not all self contained to one agency any more. Below is how they are working with the FCC and there are working agreements with the NIH.
We didn’t have all these mobile devices and sophisticated data systems a few years ago, but have them now and regulation is needed along with some programmers and in house specialists, as you can’t out source all of this as making a profit and safety have some conflicts here.
Wireless Healthcare Medical Devices and the FDA – The Reasons They Are Slow to Come to Terms
On the other side of the coin the health insurers with their machine gun Health IT technology are pushing hard to get the device out there as they save them money, but we also need good implementations so they are regulated properly and not just another era of a Big Brother type of device for control by using information received, which is what they will do with risk management assessments.
Health Insurers Expanding the Use of Devices Reporting Data – Wireless Home Units or Sensors Worn to Submit Heart Rate and Other Vital Information
They also need a bigger budget so they don’t have to keep giving grants to health insurance companies due to lack of IT technology. Back in January of 2010 with the Sentinel Initiative they found they had to outsource the colleting of safety data to an insurance company and here we go again into the “circle of profit”. 
FDA Awards a Big Grant to Health Insurance Company For Pilot Program To Monitor Safety of Drugs and Medical Devices
I have written about the Sentinel system several times on this blog and if I remember correctly, Blue Cross was one of the first health insurance companies who stated they would add data. Again, this is what we need, data for individuals at the FDA to use, not an insurance company making their recommendations based on profitability if we are in fact looking to find and create safety initiatives and programs.
Last but not least here’s my campaign again for the technology answer for recalls, hope someone wakes up soon. I’ll keep repeating this from 2 years ago is that the job of the HHS director and staff requires some IT knowledge to make these decisions and a speaker with a microphone doesn’t cut it, you need the data tools and know what you are talking about.
FDA Publishes Information on How to Identify Recalls – Why Not Scan That 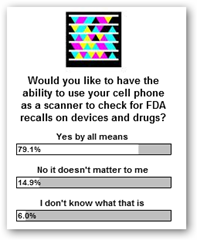 Stent With A Cell Phone and Make It Easy for the Public and Manufacturers To Keep Up, Notify And Automate Compliance
Stent With A Cell Phone and Make It Easy for the Public and Manufacturers To Keep Up, Notify And Automate Compliance
Also, there’s going to be some new unexpected areas in the FDA needing funding too that we don’t even know about now, so we need to be ready for that too, technology throws us a new left curve every day.
A year ago we didn’t have Ipads and look now, and there’s a lot of new technology that sprung up quickly and this can happen with any new technology and there’s really no control over it, but you can’t be bliss and miss opportunities either, as others won’t. BD
A group of Democrats in the House and Senate send an open letter to U.S. Dept. of Health and Humans Services secretary Kathleen Sebelius, urging her to push for a bigger boost to the Food & Drug Administration's 2012 budget.
The Aug. 4 letter was signed by Reps. Harry Waxman (D-Calif.), John Dingell (D-Mich.), Bart Stupak (D-Mich.), Frank Pallone (D-N.J.) and Sen. Tom Harkin (D-Iowa).
Congress to Sebelius: Up the ante on FDA's budget | MassDevice - Medical Device Industry News



0 comments :
Post a Comment