Read the quoted paragraph below, a veteran gets a notice 3 months after the recall,  well gee….bar codes would have made this information available immediately. The world class factory still remains closed after the FDA moved in. I did a poll and consumers want this and would use it, but nobody cares about serving the consumer, they just want their money and are acting like a BP with no disaster preparedness.
well gee….bar codes would have made this information available immediately. The world class factory still remains closed after the FDA moved in. I did a poll and consumers want this and would use it, but nobody cares about serving the consumer, they just want their money and are acting like a BP with no disaster preparedness.
One thing though is that they can sure put out a lot of useless consumer mobile software that nobody uses and some of the drug companies are retiring a lot of what they bothered with. J and J has done nothing for consumers either with all their recalls.
“At least one veteran, however, said he’s been using alcohol prep pads supplied by the VA for three years, and that officials only warned him to stop in a form letter included in a medical kit dated April 7, three months after the initial recall was issued.
Is a Non Technical FDA Recall System Like Another BP Oil Spill Waiting to Happen One Day
I have written to the FDA on this as well as drug and device companies and nothing but buy that product and bring in that money. I wrote a post about how it took me 2 weeks to get a canned response that most web sites send immediately. The White House even does that. Heck when you give the FDA the input they ask for I don’t think they know what to do with it or even if they read it.
FDA Looking for Public Input on How They Communicate With the Public-In My Case They Don’t Relative to Using Bar Codes for Device, Drug and OTC Products Recalls
By the way the President sent an executive order out asking what federal agencies are planning to improve customer service with using technology and do you they might fit this on their list…would be a good idea.
President Obama Issues Executive Order To Federal Agencies to Improve Customer Service And Use Technology To Accomplish
FDA Moves in on Triad and Seizes $6 Million Dollars of Product-The Medicated Wipe Recalls Issues Continue - Triad is an Outsource for Several Fortune 25 Companies
FDA Announces Recall of Alcohol Prep Pads, Swabs, Swabsticks From Triad–FDA and Manufacturers Should Ashamed-Campaign for Bar Codes Still Stands Stronger Than Ever!
This is by far the biggest neglect of both the FDA and drug/medical device and over the counter healthcare products!! Have you ever tried to return a product to a retail drug store and be told “no” because it’s not on their authorized list!! Now some of their products are used by healthcare professionals too and I looked all over the web site and as close as I could come was to find wipes or products that remove adhesive tape so I think this is what the product recall is all about that could be contaminated. A cell phone to scan the box would soooooo simple. 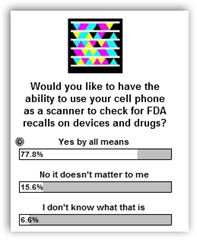
This is another good sized recall and granted the products are not expensive but for goodness sakes we have private label stuff in here now too. If in fact the products are not sanitary we need to have an easier way to find them on the shelves. Look what a mess they make for the retail stores too.
CVS and Walgreens should join my cause here as this is just simply “tech denial” and nobody wanting to do a thing about it. I have sent this to drug companies, the FDA, the DEA and several other deaf ears. This is costing the retail stores money too! Again, a good reason for them to join the cause here.
I just did a post this morning about a company that uses bar codes to help us determine what nutritional values sit on the grocery shelves so everyone wants us to eat right but if something is recalled, go ahead and get sick and die is the message I am hearing. The same thing with Walgreens, only efforts go out that generate money and nothing for consumer safety and information and pick up some data for sale on you when you use the bar code.
Walgreens Releases Windows 7 Application Using Bar Code Technology To Order Prescription Refills
All involved need to collaborate here and fix this and get those bar codes out to help consumers. My little campaign is getting close to 2 years old now and has permanent links at the top of the page. BD
A quarter of the nation’s Veterans Health Administration medical centers and the agency's outpatient mail-order pharmacy used recalled alcohol prep pads and other products blamed in lawsuits for infections and a death, msnbc.com has learned, prompting a new round of concerns from a U.S. senator.
Bennet, along with Sen. Lamar Alexander, R-Tenn., had previously questioned the federal Food and Drug Administration’s handling of problems with contamination and sterilization at the Wisconsin plant. Those issues eventually led to the recall of hundreds of millions of products used in hospitals, clinics and homes and widely sold in grocery and drug stores.
Thirty-eight of the country’s 152 major veterans medical centers in 30 states and the District of Columbia removed recalled wipes, pads and other products from use, VA officials told msnbc.com. See the box below for a complete list. In addition, the products were removed from the Consolidated Mail Outpatient Pharmacy, which provides more than 97.4 million prescriptions a year to veterans.
The original recall has expanded 40 times since it was issued in January, Hammonds wrote, as Triad Group products were detected in other products such as suture kits that involved additional brand names.
Suspect wipes used at VA medical centers - Health - Infectious diseases - msnbc.com



0 comments :
Post a Comment