This is not surgery to make your lets longer, that’s another topic and company but  rather this is rather an orthopedic device to remotely control implants in your legs for bone lengthening. If you want to read about surgery to be taller and all the pain and suffering and bone breaking, then check out the link below.
rather this is rather an orthopedic device to remotely control implants in your legs for bone lengthening. If you want to read about surgery to be taller and all the pain and suffering and bone breaking, then check out the link below.
Surgery to Become Taller – Cosmetic Limb Lengthening (CLL)
Spinal scoliosis in children is the first targeted market and that sounds like a very good place to begin. The company is located in Irvine, California and the device already has the European CE mark of approval.
“The pictures are pretty interesting though and it looks kind of sci-fi looking if you will or like a big hardware type of tool. From the website below: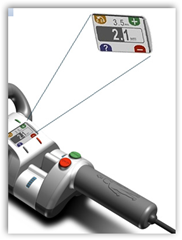
Our initial products are non-invasively adjustable implants used to restore proper anatomic position and alignment of the spine and long-bones. The key to Ellipse Technology is the non-invasive magnetic interaction between the implant and the External Remote Controller “ERC”, a portable, hand held unit that uses permanent rare earth magnets to automatically modify the length of the implant through the touch of a switch. The amount of lengthening or shortening of the MAGEC or PRECICE implant can be seen immediately on the ERC display module.”
This seems pretty wild as you use the device to automatically modify the length of the implant with a switch on the hand held unit and I can guess this is going to cost a few dollars too when available. BD
Press Release:
IRVINE, Calif.--(BUSINESS WIRE)--Ellipse Technologies, Inc. (“Ellipse”) announced today that it has received FDA marketing clearance of the Company’s PRECICETM Limb Lengthening device in the United States. Limb Lengthening procedures are used to treat a number of medical conditions, including legs shortened due to congenital abnormalities, major fractures of one of the legs and shortened leg bones due to other medical diseases, such as cancer.
“Our remote control technology was a huge hit among attendees. The PRECICE System is easily recognized as a game-changer for patients suffering from limb deformities”
Ellipse has initiated clinical use of the PRECICE devices and plans an international product launch during the first half of 2012.
Commenting on the PRECICE technology, Stuart Green, M.D., Professor of Orthopedic Surgery, University of California, Irvine, said, “The PRECICE Technology will make it possible to use externally controllable implants for patients who require bone lengthening. In the future, this technology will likely be adapted to many other orthopedic applications.”
PRECICETM Remote Control Limb Lengthening System
The initial PRECICE devices will be used in leg limb lengthening procedures of the femur and tibia bones. Rather than using adjustable external fixation systems which are attached to the leg bone through long-term openings in the skin, the PRECICE REMOTE CONTROL TECHNOLOGY provides an internal implant adjusted to lengthen the leg bones via non-invasive methods from outside the body. Ellipse and its scientific advisors believe the PRECICE devices will not only provide a less-invasive approach to these procedures but also significantly reduce the potential for complications (e.g., infections) during the healing and recuperation period.
The PRECICE System was recently unveiled at the Limb Lengthening and Reconstruction Society (LLRS) Annual Meeting in Chicago. “Our remote control technology was a huge hit among attendees. The PRECICE System is easily recognized as a game-changer for patients suffering from limb deformities,” said Ed Roschak, Ellipse Chief Operating Officer.
Ellipse is continuing to develop the PRECICE technology for orthopedic fracture management and trauma applications.
MAGECTM Remote Control Spinal Deformity System
Ellipse has developed the MAGEC (MAGnetic Expansion Control) Technology for minimally invasive, and ultimately non-invasive, orthopedic deformity prevention and management. MAGEC Technology is a breakthrough medical device technology capable of non-invasively adjusting implants within the human body from outside the body via remote control. The adjustment of the device can also be reversed. The first application for this technology is for the treatment of spinal scoliosis in children.
With the MAGEC Technology, a single minimally invasive surgical procedure is completed. Then, during a series of routine outpatient visits, the physician will dynamically adjust the MAGEC Technology from outside the body via the MAGEC System’s External Remote Controller (“ERC”), thus eliminating the need for multiple highly invasive surgical procedures which are required with currently marketed, conventional products.
The MAGEC System is CE-Marked and Ellipse recently initiated a product launch at the International Meeting for Advanced Spine Therapies (IMAST) in Copenhagen, Denmark. Commenting on this launch, Mr. Roschak said, “The response to MAGEC from the international spine community at IMAST was profoundly positive. The vast majority of physicians told us the Ellipse breakthrough technology will be of great benefit to their patients with spinal deformity. Now, we can move forward with the international rollout of the MAGEC System.”
Ellipse Technologies, Inc. is a privately-held medical device company located in Irvine, California. The Company is focused on developing its implantable remote control technology platforms to include innovative and state-of-the-art treatments for a broad spectrum of spinal and orthopedic deformity applications, orthopedic trauma and fracture management.
The MAGECTM System is not currently available for distribution in U.S.





0 comments :
Post a Comment