No this does not talk to your cell phone, it has it’s own device that it talks to. Once implanted the physician can review the pulmonary artery pressure and the heart rate. Patients must have been hospitalized for heart failure before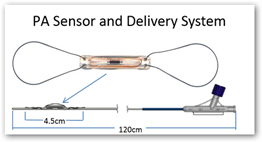 the unit can be used. BD
the unit can be used. BD
“The sensor is a resonant circuit consisting of a capacitor and an inductor. The capacitance of the sensor is a function of the pressure in the sensor's environment and the resonant frequency of the sensor is a function of the capacitance and is measured by the electronics unit. Because of the presence of the inductor coil, the sensor can be electromagnetically coupled and the resonant frequency of the LC (inductor-capacitor) circuit can be measured remotely. This allows for wireless communication with the sensor and eliminates the need for a battery. “
The sensor is implanted in a branch of the left or right pulmonary artery and remains in the pulmonary artery as a permanent implant. The sensor is tethered to an over-the-wire delivery catheter.
The Food and Drug Administration on Wednesday approved a new implantable device to help doctors monitor
 patients with severe heart failure as they go about their day. The agency cleared the CardioMEMS HF System for patients who hospitalized in the previous year because of heart failure. The device uses an implanted sensor in the peripheral artery to measure blood pressure and heart rate. The information is wirelessly transmitted to an electronic database that can be retrieved by patients’ physicians. The F.D.A. cleared the device based on a study of 550 patients in which those with the device had significantly fewer heart-failure-related hospitalizations than those without it
patients with severe heart failure as they go about their day. The agency cleared the CardioMEMS HF System for patients who hospitalized in the previous year because of heart failure. The device uses an implanted sensor in the peripheral artery to measure blood pressure and heart rate. The information is wirelessly transmitted to an electronic database that can be retrieved by patients’ physicians. The F.D.A. cleared the device based on a study of 550 patients in which those with the device had significantly fewer heart-failure-related hospitalizations than those without it



0 comments :
Post a Comment