This is a big even in the move towards personalized medicine. This test will allow the physician to determine if the use of Herceptin is the right treatment for the patient with breast cancer! If it is determined not to work, then the patient is spared the expense and use of the drug, and better yet, if the test shows it is useful, then a treatment plan can begin. This should also help with getting the insurance company to pay as well. The process can be done in most hospital labs according to the article, without any special equipment to be purchased. BD 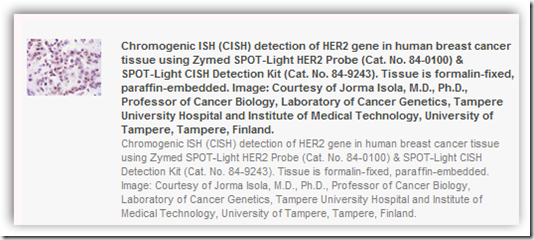
Invitrogen has won FDA approval of a test that can determine whether a woman with breast cancer will benefit from Genentech's Herceptin. The SPOT-Light test, as it is known, uses a DNA probe for the HER2 gene, which is amplified in 18 to 30 percent of breast cancers and can predict whether a patient will benefit from Herceptin. While a test is already available for Herceptin, many smaller hospitals don't have the resources to process the tests. Invitrogen's SPOT-Light doesn't require specialized equipment and can be processed in most hospital labs.



0 comments :
Post a Comment