This is a smaller knee implant that does not require as much shaving of the bone, resurfacing instead. The site has some nice videos to show how it compares to a total knee implant.
The products are FDA approved. What is nice is that a CT scan is done and the image is uploaded to their imaging service, which uses the specifications to create the device, so it is a full custom fit from the start and the product is also suitable for young adults as well. BD 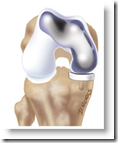
BURLINGTON, Mass., Aug 04, 2008 (BUSINESS WIRE) -- ConforMIS, Inc., a privately held orthopedics company that develops and commercializes minimally invasive medical devices for the treatment of osteoarthritis and joint damage, today announced the launch of the first and only patient-specific bicompartmental knee resurfacing implant on the market. The iDuo(TM) is the third in the line of minimally invasive, patient-specific implants.
ConforMIS has developed for the treatment of osteoarthritis of the knee, the most common reason for knee replacement surgery.



0 comments :
Post a Comment