Recalls of both medical devices and drugs are growing for a number of reasons. First of all, we have a lot more information available today than what we have  ever had and we need to capitalize on this opportunity quickly. We read in the news every day it seems about quality control issues, devices needing software updates and so on. How do we get the word out quickly and efficiently? If one has times they can certainly search the web and put out a full on effort to find all of this every day, but healthcare workers have the same problems we all have and that is time. When human lives are involved, time is everything.
ever had and we need to capitalize on this opportunity quickly. We read in the news every day it seems about quality control issues, devices needing software updates and so on. How do we get the word out quickly and efficiently? If one has times they can certainly search the web and put out a full on effort to find all of this every day, but healthcare workers have the same problems we all have and that is time. When human lives are involved, time is everything.
The opportunity to turn a cell phone into a “scanner” with real time information is huge. As mentioned above, this can be a daunting task at times and we have people at all different stages with using technology today and in my opinion, using a cell phone makes sense, when all one has to do is open a program on the phone and simply “shoot and aim” and relative information would be available instantly. Back in October of 2009 I kept reading about all the recalls of devices and created my first opinion/idea post here.
Microsoft Tags – Microsoft MSDN Posts Ideas from the Medical Quack About Use in Healthcare!
It would certainly be a lot easier to scan and not have to look up all the Lot numbers, you think? BD 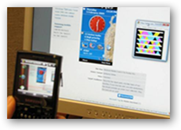
FOR IMMEDIATE RELEASE -- July 12, 2010 - Bristol-Myers Squibb initiates a voluntary recall of 3 lots of physician sample blister packs of Coumadin® 1 mg tablets and 5 lots, of Coumadin 1 mg tablet hospital unit dose (HUD) blister packs.
The following lot numbers are included in this recall: Physician Sample Blister Packs: Lot# 9A48931A, 9A48931B, 9A48931C, expiration January 2012; HUD Blister Pack: Lot# 8F34006B, 8K44272A, 8K46168A, 9F44437A and 9K58012B with expiry dates between June 2011 and November 2012.
The recall is a precautionary measure based upon the company’s determination that some of the tablets, over time, may not meet specification for isopropanol. Isopropanol is used to maintain the active ingredient, Coumadin, in the crystalline state, and could affect the therapeutic levels of the active ingredient.




0 comments :
Post a Comment