It doesn’t matter why the recall is being done, but whether it is a consumer, a pharmacy, pharmacy benefit manager or a hospital, here goes the long pain-staking process once again and stuff can get missed too. I’m not going to elaborate again  and re-type the solution but will copy and paste a solution. Some nice heat maps to find the drugs when scanned would be be very cool.
and re-type the solution but will copy and paste a solution. Some nice heat maps to find the drugs when scanned would be be very cool.
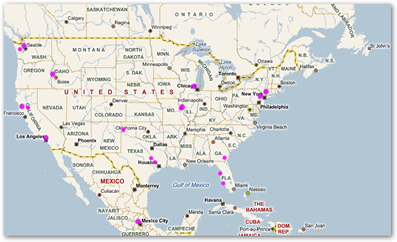
But we don’t choose to investigate this and still drive individuals to the long pain staking process of looking up lot numbers etc. when a simple cell phone scan could do the trick. My bar code campaign would be an effort of the FDA, DEA and drug and device companies working together and everyone benefits if collaboration were able to be facilitated, the key to data resolutions today. Here’s my heat map where I can see who has scanned my Tags (bar codes). Pretty neat and in real time.
Abbott Recalls Certain Similac® Brand Powder Infant Formulas–Bar Codes and Heat Map Technology Would Have Made the Process Easier-Scan for Recalls with Cell Phones
I just read over at the Wall Street Journal the Abbot Recall Site and Hotlines are over whelmed so be prepared to wait. In the meantime, there’s other technologies like Microsoft Tags that could have (if used) enabled consumers the opportunity to scan for recalled products by using their cell phone. If you like this idea, give it a positive vote on the poll. There’s a long write up summary on the image above and it can be found at the top of my blog where it has a permanent resting spot.
Microsoft Tag Bar Codes–Who’s Been Scanning the Medical Quack–The Bing Heat Map Tells All And Could Help Find Stolen or Expired Drugs and Devices With This Methodology
There will be more recalls in both food, drug and device products so we need a system that can work for everyone and especially the consumer as we are the ones affected. When you look at this map, and when products were scanned, guess what, it could give Abbott even more intelligence in knowing where the product was due to “spots” on the map so they in turn could be more pro-active here too with finding the product quicker! What technology that is available today for implementation can do and save lives too.

I’m actually surprised to see investors not demanding a better recall system too as this affects their profits and of course we know Wall Street uses the most coveted and highest performing technology with data and algorithms available today. BD
The recall of the Puerto Rico-manufactured drugs is not expected to disrupt the availability of Epogen to patients, according to Amgen spokeswoman Emma Hurley.
She also said the biotechnology company does not expect a material financial impact from the recall.
Amgen manufactures Epogen, used mostly for kidney dialysis patients, and Procrit, used mostly for cancer patients. Amgen sells Epogen in the United States and J&J's Centocor Ortho Biotech unit distributes Procrit in the United States. J&J has been dealing with a rash of recalls of its consumer medicines.
Amgen, J&J anemia drugs recalled over glass flakes | Reuters



0 comments :
Post a Comment