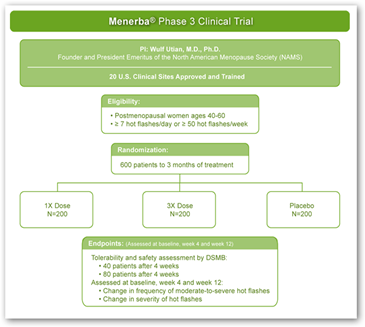
The trial will be fore 600 postmenopausal women who experienced at least 7 moderate to severe hot flashes per day or at least 50 moderate to severe hot flashes per week. They want some serious flashers here for this one. Information has also been posted on the website for additional information. Trials and centers are or will be located in Alabama, Arizona, California, Idaho, Iowa, Maryland, Massachusetts, Minnesota, New Jersey, New York, Ohio, Pennsylvania, Tennessee and Utah. BD
Bionovo Inc. (BNVI) said the Food and Drug Administration indicated its agreement with a plan for Phase III clinical testing of Menerba, its drug to treat hot flashes.
Shares jumped 12%, or 5 cents, to 43 cents in late trading.
About 40 million women in the U.S. and 37 million in Europe suffer from hot flashes and other symptoms associated with menopause. The only FDA-approved drugs for the symptoms are hormone-replacement therapies with serious side effects, including breast cancer.
Menerba's Phase II clinical trial showed it is well tolerated with no serious side effects and is statistically superior to placebos in reducing the number of hot flashes in menopausal women.
Bionovo is now in final discussions with the FDA to complete the design of the Phase III trial in the U.S. When the design is finalized, enrollment in the trial will follow.
Bionovo: FDA OKs Plan For Trials Of Hot-Flashes Drug Menerba - WSJ.com




0 comments :
Post a Comment